Introduction
Stability is one of the most critical factors in pharmaceutical formulations, food products, and industrial suspensions. A stable formulation ensures the product’s safety, efficacy, and quality throughout its shelf life. However, various factors can lead to instability, causing degradation, sedimentation, and phase separation.
Understanding stability issues and implementing strategies to prevent them can help manufacturers develop reliable pharmaceutical formulations with optimal performance. This article will explore the common stability problems in suspensions, their causes, and methods to overcome them.
What is stability in pharmaceutical formulations?
Pharmaceutical stability refers to a drug’s ability to maintain its physical, chemical, microbiological, and therapeutic properties over time under specified conditions. Stability testing is essential to determine shelf life and ensure compliance with regulatory standards.
Common Stability Problems in Pharmaceutical Suspensions
1. Physical Instability
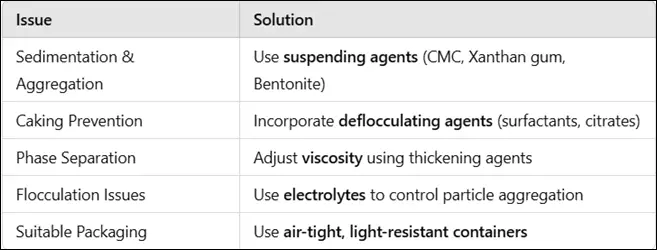
Physical instability in pharmaceutical formulations can lead to visible changes such as phase separation, sedimentation, and aggregation. These issues affect drug uniformity, making it difficult to achieve accurate dosing.
1.1 Sedimentation & Aggregation
- Occurs when solid particles settle at the bottom due to gravity.
- Leads to non-uniform drug distribution in suspensions.
1.2 Caking (Hard Sediment Formation)
- Over time, particles form a dense cake at the bottom.
- Hard sediment is difficult to redisperse, making the suspension ineffective.
1.3 Phase Separation (Creaming and Coalescence)
- The liquid medium and suspended particles separate over time.
- Can be seen as an oily layer or clear liquid above the sediment.
1.4 Flocculation & Deflocculation
- Flocculated suspensions: Particles form loose aggregates, settling quickly but redisperse easily.
- Deflocculated suspensions: Particles remain separate, settling slowly but forming a hard cake.
2. Chemical Instability
Chemical instability results from interactions between the active pharmaceutical ingredient (API) and excipients or external environmental factors.
2.1 Hydrolysis (Water-Induced Degradation)
- Some drug molecules break down in the presence of moisture.
- Example: Aspirin hydrolyzes into salicylic acid and acetic acid.
2.2 Oxidation
- Oxygen exposure leads to the degradation of sensitive drugs.
- Example: Vitamin C degrades when exposed to air.
2.3 Photodegradation
- Light-sensitive drugs degrade upon exposure to UV light.
- Example: Riboflavin and some antibiotics.
2.4 pH Drift
- pH fluctuations can lead to instability in solutions and suspensions.
- Example: Changes in pH can reduce drug solubility.
3. Microbial Instability
Contamination by bacteria, fungi, and other microorganisms can compromise pharmaceutical products.
3.1 Bacterial & Fungal Growth
- Microbes multiply in aqueous formulations without preservatives.
- Causes spoilage and potential infections.
3.2 Biofilm Formation
- Some microorganisms form protective biofilms, making preservatives ineffective.
How to Overcome Stability Problems in Pharmaceutical Formulations
1. Enhancing Physical Stability
1.1 Use of Suspending Agents
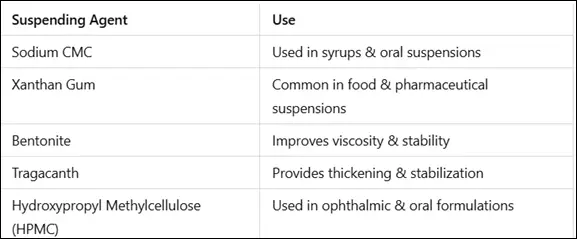
- Prevents sedimentation and maintains uniform drug distribution.
- Examples: Carboxymethylcellulose (CMC), Xanthan gum, Bentonite.
1.2 Optimization of Particle Size
- Reducing particle size improves stability and dispersion.
- Methods: Micronization, Wet Milling, Nanoparticle Technology.
1.3 Incorporation of Deflocculating Agents
- Helps maintain separate particles and prevents caking.
- Examples: Surfactants, Citrates, Polyphosphates.
1.4 Adjustment of Viscosity
- High-viscosity formulations prevent phase separation.
- Agents: Polyvinyl alcohol, Hydroxypropyl methylcellulose (HPMC).
1.5 Use of Suitable Packaging
- Airtight, moisture-proof, and UV-resistant containers improve stability.
- Examples: Amber glass bottles, blister packs.
2. Improving Chemical Stability
2.1 Use of Antioxidants
- Prevents oxidation and degradation.
- Examples: Ascorbic acid, butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA).
2.2 pH Control with Buffers
- Helps maintain a stable environment.
- Examples: Citric acid, sodium phosphate.
2.3 Light-Resistant Packaging
- Protects photodegradable drugs.
- Examples: Amber Glass, UV-Blocking Films.
2.4 Moisture Protection
- Reduces hydrolysis by using desiccants.
- Example: Silica gel, calcium chloride.
3. Enhancing Microbial Stability
3.1 Use of Preservatives
- Prevents bacterial and fungal contamination.
- Examples: parabens, benzalkonium chloride, sorbic acid.
3.2 Sterile Manufacturing Conditions
- Ensures contamination-free production.
3.3 Refrigeration for Storage
- Helps prolong shelf life for sensitive formulations.
3.4 Proper Packaging Techniques
- Sealed packaging prevents microbial invasion.
Best Suspending Agents for Liquid Formulations
Suspending agents improve the dispersibility of particles and prevent settling.
How to Prevent Caking in Suspensions
To prevent hard sedimentation (caking), use:
- Deflocculating agents like surfactants
- Electrolytes to control particle charge
- Viscosity enhancers to reduce settling
- Proper agitation during formulation
Pharmaceutical Suspension Stability Testing
Stability tests ensure formulations remain effective and safe over time.
Types of Stability Testing:
- Accelerated Stability Testing – Exposing formulations to extreme conditions (high temperature, humidity) to predict long-term stability.
- Real-Time Stability Testing – Observing stability under normal storage conditions over time.
- Microbial Stability Testing – Checking for Bacterial & Fungal Contamination.
Conclusion
Stability problems in pharmaceutical suspensions can significantly impact product effectiveness and patient safety. However, by incorporating proper suspending agents, antioxidants, preservatives, and packaging strategies, these challenges can be overcome. Understanding pharmaceutical stability helps in formulating better drugs with longer shelf life and consistent efficacy.
Frequently Asked Questions (FAQs)
1. What are the common stability problems in pharmaceutical suspensions?
Answer: Common stability problems include sedimentation, caking, oxidation, microbial growth, and phase separation.
2. How can caking be prevented in suspensions?
Answer: Using deflocculating agents, suspending agents, and particle size optimization helps prevent caking.
3. What role do antioxidants play in stability?
Answer: Antioxidants like BHT, BHA, and ascorbic acid prevent oxidation, preserving drug potency.
4. How do preservatives enhance stability?
Answer: Preservatives such as parabens and benzalkonium chloride inhibit microbial growth, ensuring longer shelf life.
5. Why is pH control important for formulation stability?
Answer: pH influences drug solubility, degradation, and overall formulation stability.