The various physiochemical properties affecting the drug action are-
1. Ionization 5. Protein bonding
2. Solubility 6. Chelation
3. Partition coefficient 7. Bioisosterism
4. Hydrogen bonding 8. Stereoisomerism
Ionization:
It is necessary to know whether a molecule will be ionized at a given pH simply by knowing if the functional groups on the molecule area are acidic or basic. The Henderson-Hassalbach equation can be used to calculate the % ionization of a compound at a given PH.
In the case of acid,
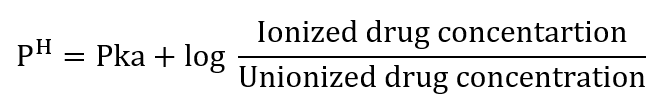
In the case of the base,
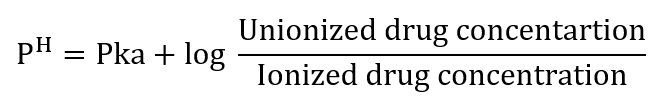
The biological activity of certain acids and bases is directly related to their degree of ionization. Whereas some (e.g. phenols, carboxylic acids) act in the molecular form, others (quaternary ammonium salts) act in an ionized form. In these cases, the pH plays an important role; acids are more active at lower pH, and bases are more active at higher. .
- A strong acid has a low pKa value.
- A weak acid has a high pKa value.
- A strong base has a high pKa value.
- A weak base has a low pKa value.
Solubility:
The solubility of a drug in polar solvents and non-polar solvents depends upon various factors such as structure, particle size, surface, and crystal form. Polar non-ionic compounds form hydrogen bonds with water lipids and get dispersed.
Partition coefficient:
It is an important property that affects a drug molecule’s biological action. It is defined as the ratio of the concentration of a drug molecule in the lipid phase and water phase.
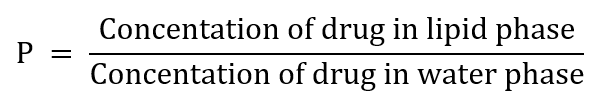
Hydrogen bonding:
A bond in which a hydrogen atom holds other atoms together as a hydrogen bond (H-bond). This bond is formed between hydrogen and electronegative atoms. The higher the hydrogen bonds, the more water solubility. Each intermolecular hydrogen bond decreases water solubility and increases lipid solubility. The H-bond significantly alters a compound’s physical, chemical, and biological properties. The most common atoms capable of forming H-bonds are F, O, N, and to a lesser extent Cl and S. Generally, hydrogen bonding is classified into two types:
- Intermolecular hydrogen bonding
- Intramolecular hydrogen bonding
Intermolecular hydrogen bonding: Hydrogen bonding occurs between two or more molecules
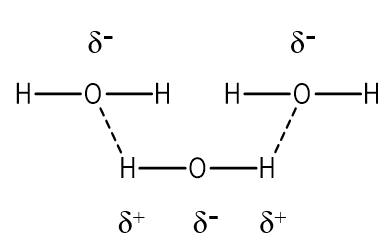
Intramolecular hydrogen bonding: Hydrogen bonding occurs within the molecules
There is also evidence that the hydrogen attached to a triple-bond carbon (e.g. HCN and CHCl3) forms an H-bond. H-bonds strength ranges from 1 to 10 kcal/mol and is usually about 5 kcal/mol. The distance between the electronegative elements in a H-bond is usually in the range of 2.5–2.7 A°. At a distance greater than 3 A°, there is very little interaction (Table 3.1). H-bonds’ stability falls roughly in the following order, OHO > OHN > NHN.
Table H-bond and its bond strength.
| H-bond | Bond Strength (kcal/mol) |
| F-H—-F | 7 |
| O-H—-O | 4.5-7.6 |
| O-H—-N | 4-7 |
| C-H—-pi electrons | 2-4 |
| C-H—-O | 2-3 |
| N-H—-O | 2-3 |
| N-H—-N | 1.3 |
Since a compound’s physical and chemical properties may be greatly altered by hydrogen bonding, it is reasonable to expect that it may also have a significant effect and some correlation with biological properties. In a number of cases, such a correlation is present (Tables 3.2 and 3.3).
Table 3.2 Differences between 1-phenyl-3-methy-5-pyrazolone and 1-phenyl-2, 3-dimethy-5-
pyrazolone (antipyrine).
| 1-Phenyl-3-methy-5-pyrazolone | 1-Phenyl-2,3-dimethy-5-pyrazolone (antipyrine) |
| Less analgesic property | Good analgesic property |
| Melting point 127°C | Melting point 122°C |
| Slightly soluble in water | Soluble in water |
| Forms intermolecular hydrogen bonding | Does not form intermolecular hydrogen bonding |
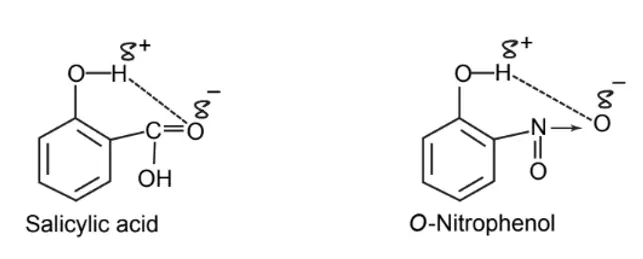
Table: Differences between o-hydroxybenzoic acid and p-hydroxybenzoic acid.
| o-Hydroxybenzoic acid (Salicylic acid) | p-Hydroxybenzoic acid |
| pKa.3.0 | pKa.4.5 |
| Less soluble in water | More soluble in water |
| Low melting point | High melting point |
| Good antibacterial action | Low antibacterial action |
| Forms intramolecular hydrogen bonding | Forms intermolecular hydrogen bonding |
Protein bonding:
The drug in the body interacts with various components like blood and extravascular tissues. This binding of drugs with proteins (macromolecules, blood, etc.) is of two types-
- Intracellular binding.
- Extracellular binding.
The protein binding of drugs helps in drug absorption, solubility, and distribution.
Chelation:
Chelation is a chemical process in which a substance is used to bind molecules, such as metals or minerals, and hold them tightly. Chelation has been used to rid the body of excess or toxic metals. It has some uses in conventional medicine, such as treating lead poisoning or iron overload.
“Chalets are basically ring structures formed between complex agents and the metal ion.” Hemoglobin and cyanocobalamine are naturally occurring chalets. For example, EDTA, dimethylglyoxime, and Dimercaprol (used in arsenic poisoning).
Bioisosterism:
Bioisosters are substituents or groups with similar physical or chemical properties that produce broadly similar biological properties to other chemical compounds. The relation between bioisosteres is known as bioisosterism
In drug design, exchanging one bioisoster for another enhances a compound’s desired biological or physical properties without making significant changes in chemical structure.
Langmuir first developed the concept of chemical disasters to describe the physical properties of atoms, functional groups, and molecules. A compound or group of atoms having the same number of atoms and electrons.
Burger defines bioisosters as substituents or groups with similar chemical and physical properties that produce broadly similar biological properties.
Bioisosters are used to reduce toxicity or modify the activity of a lead compound and may alter the metabolism of the lead. Bioisosters have the same number of atoms and the same number of electrons; the electrons may arrange themselves in the same manner.
Stereoisomerism:
Stereoisomerism involves the study of the three-dimensional nature of molecules. It is the study of the chiral molecules.
- Stereochemistry plays a major role in the pharmacological properties because any change in the stereospecificity of the drug will affect its pharmacological activity.
- The isomeric pairs have different physical properties (log p, pKa, etc.) and thus differ in pharmacological activity.
- The isomers with the same bond connectivity but different arrangements of groups or atoms in space are termed stereoisomers.
Optical isomerism arises due to differences in plane-polarised light rotation by the drug molecule, i.e., the drug may be (+) dextrorotatory or (-) levorotatory. Various examples are-
Both enantiomers (+) and (-) have different biological activities.
- (-) adrenaline in more active then (+) adrenaline.
- (-) Warfarin is 5 times more potent than (+) isomer.
- (-) Propranolol is more potent than (+) propranolol.
- (+) Amphetamine is 3-4 times more active than (-) isomer.
In certain cases, one isomer is active while the other isomer is toxic. For example-
D-Penicillamine is used in the treatment of arthritis while L-Penicillamine is highly toxic.
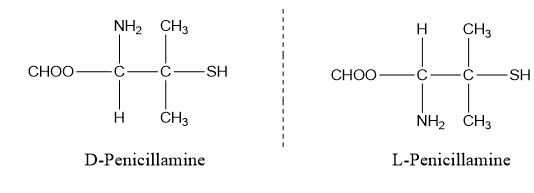
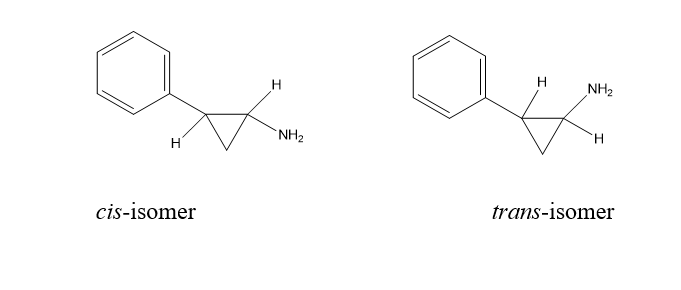
Geometrical Isomerism:
This isomerism is represented by cis and trans isomerism. The isomerism or arrangements of atoms around the C=C bond. It occurs as a result of or due to the presence of (C=C).
For example-
- Trans-diethyl stilbestrol is more potent than Cis-isomer, i.e., higher estrogenic activity.
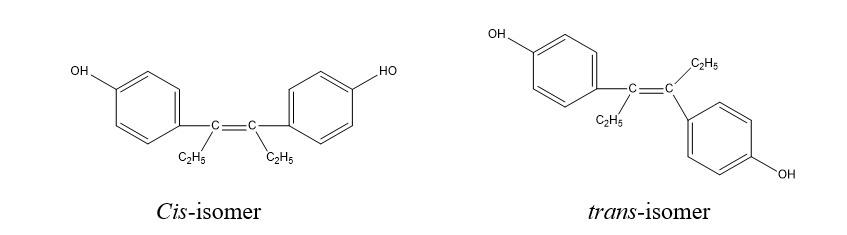
2. Cis-2-phenylcyclopropylamine is more active as an inhibitor of Monoamine oxides (MAO) than its trans-isomer.