Introduction
Acid-base balance is essential for maintaining the body’s pH homeostasis, ensuring that enzymes, cellular processes, and metabolic functions operate efficiently. The normal blood pH range is 7.35–7.45, regulated by buffer systems, respiratory control, and renal mechanisms.
Any imbalance in pH can lead to acidosis (low pH) or alkalosis (high pH), affecting cellular function, enzyme activity, and overall metabolism.
In this post, we will explore the physiological mechanisms of acid-base balance, key buffer systems, and clinical significance.
What is Acid-Base Balance?
Acid-base balance refers to the body’s ability to regulate hydrogen ion (H⁺) concentration to maintain a stable pH (7.35–7.45).
Why is it Important?
1. Ensures proper enzyme function.
2. Maintains nerve and muscle activity.
3. Supports oxygen transport and metabolism.
pH Scale and Acid-Base Imbalance:
- (a) Acidosis (pH < 7.35): Excess H⁺ ions, lead to CNS depression, coma, and respiratory failure.
- (b) Alkalosis (pH > 7.45): Excess OH⁻ ions, causing muscle spasms, confusion, and cardiac arrhythmias.
Major Physiological Buffer Systems
The body maintains acid-base balance through three major buffer systems that neutralize H⁺ and OH⁻ ions.
1. Bicarbonate Buffer System (HCO₃⁻ / H₂CO₃)
Location: Extracellular fluid (ECF) and blood plasma.
Function: Maintains pH stability in blood and tissues.
Mechanism:
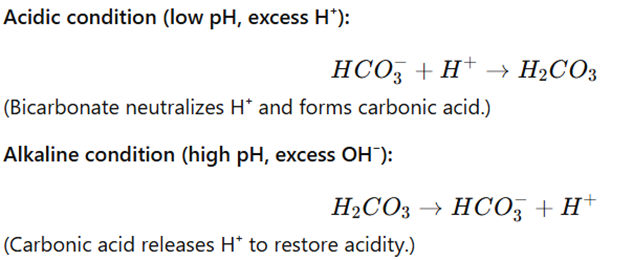
Clinical Importance:
- Metabolic acidosis (low HCO₃⁻): Seen in kidney failure, diarrhea, and diabetic ketoacidosis (DKA).
- Metabolic alkalosis (high HCO₃⁻): Occurs in excess vomiting or bicarbonate overdose.
2. Phosphate Buffer System (H₂PO₄⁻ / HPO₄²⁻)
Location: Intracellular fluid (ICF) and kidneys.
Function: Maintains pH in cells and urine.
Mechanism:
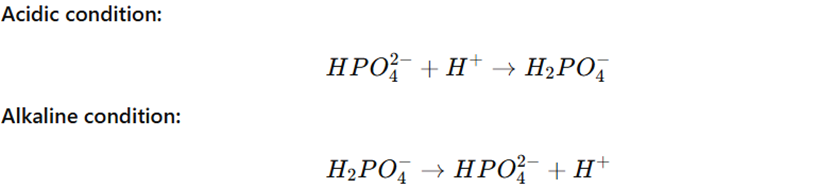
Clinical Importance:
- Plays a critical role in kidney function, regulating urine pH and excreting excess acids.
3. Protein Buffer System (Hemoglobin and Albumin)
Location: Blood, cells, and plasma proteins.
Function: Regulates pH by binding or releasing H⁺ ions.
Mechanism:
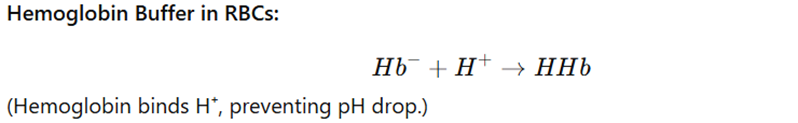
Clinical Importance:
- Respiratory acidosis: Caused by CO₂ buildup in conditions like COPD and hypoventilation.
Respiratory Regulation of Acid-Base Balance
Lungs regulate pH by controlling CO₂ levels.
Equation:
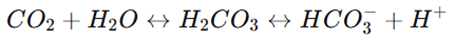
How it works:
- Increased CO₂ (Hypoventilation) → Acidosis
- Decreased CO₂ (Hyperventilation) → Alkalosis
Clinical Conditions:
- Respiratory acidosis: Seen in asthma, COPD, and lung disease.
- Respiratory alkalosis: Caused by hyperventilation, anxiety, and fever.
Renal (Kidney) Regulation of Acid-Base Balance
Kidneys maintain pH by:
- Excreting H⁺ ions (acid removal).
- Reabsorbing HCO₃⁻ (bicarbonate conservation).
Clinical Conditions:
- Metabolic acidosis: Occurs in renal failure (kidneys can’t excrete H⁺).
- Metabolic alkalosis: Seen in excess bicarbonate retention (antacid overdose).
Acid-Base Disorders and Compensation
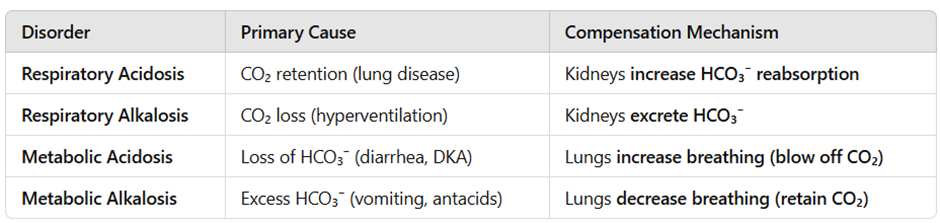
Example:
- Diabetic Ketoacidosis (DKA) → Causes metabolic acidosis.
- Lungs compensate by hyperventilation (Kussmaul breathing) to blow off CO₂.
Importance of Acid-Base Balance in Health
- Ensures enzyme function and metabolic stability.
- Prevents neurological disorders caused by pH imbalance.
- Essential for cardiovascular and respiratory health.
- Maintains homeostasis in vital organs (brain, kidneys, lungs).
Example:
- 1. Acidosis can depress the central nervous system (CNS), leading to coma.
- 2. Alkalosis can cause muscle twitching and seizures.
Conclusion
The physiological acid-base balance is maintained by buffer systems, respiratory control, and renal mechanisms to keep blood pH between 7.35 and 7.45.
✔ Bicarbonate, phosphate, and protein buffers neutralize pH fluctuations.
✔ Lungs regulate CO₂ to control acidity.
✔ Kidneys excrete H⁺ and conserve HCO₃⁻ for long-term pH stability.
Understanding acid-base balance helps diagnose and treat respiratory and metabolic disorders, ensuring optimal physiological function.
FAQs
1. What is the most important buffer system in the body?
Ans: The bicarbonate buffer system (HCO₃⁻/H₂CO₃) is the primary buffer for blood pH regulation.
2. How do the kidneys help maintain pH balance?
Ans: The kidneys excrete hydrogen ions (H⁺) and reabsorb bicarbonate (HCO₃⁻) to regulate blood pH.
3. What happens in respiratory acidosis?
Ans: CO₂ buildup (e.g., lung disease) lowers pH, leading to CNS depression.
By understanding physiological acid-base balance, healthcare professionals can prevent complications from pH imbalances and optimize patient health.



