Introduction
Potentiometry is an electroanalytical technique used to measure the electrical potential (voltage) of an electrochemical cell without drawing any significant current. This method is widely used to determine the concentration of ions in a solution using ion-selective electrodes (ISEs), such as the pH electrode.
Potentiometry is based on the Nernst equation, which relates the electrode potential to the concentration of the analyte. It is widely applied in pharmaceutical analysis, environmental monitoring, food industry, and clinical diagnostics.
Basic Principles of Potentiometry
- The electrode potential is measured under zero-current conditions to avoid disturbing the system.
- The potential of the electrochemical cell is given by the equation:
Ecell = Eindicator − Ereference
Where:
Ecell = Measured potential of the cell
Eindicator = Potential of the indicator electrode (which responds to the analyte)
Ereference = Potential of the reference electrode (constant)
- The relationship between electrode potential and ion concentration is described by the
Nernst equation
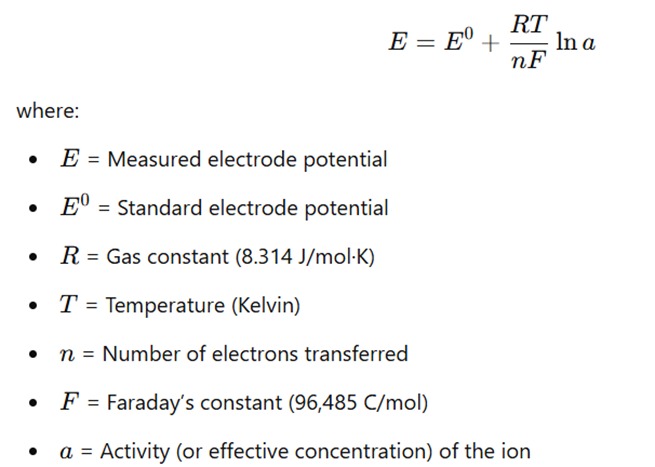
- At 25 °C, the equation simplifies to:

This equation shows that the measured potential is directly related to the logarithm of the ion concentration.
Electrodes Used in Potentiometry
1. Reference Electrodes
Reference electrodes provide a stable potential against which the potential of the indicator electrode is measured. Common reference electrodes include:
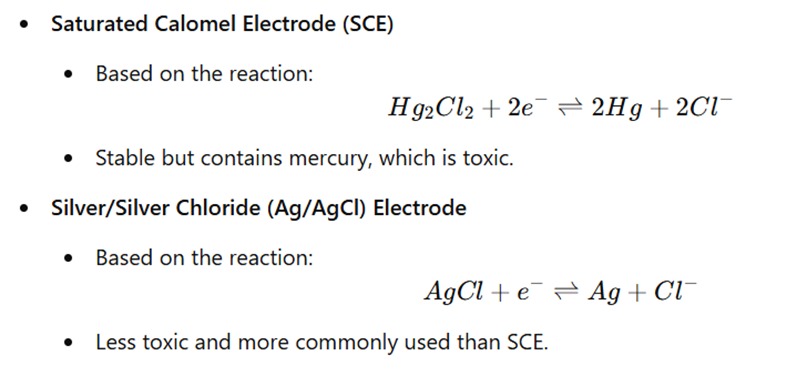
2. Indicator Electrodes
Indicator electrodes respond to the concentration of a specific ion in the solution.
a. Metal Electrodes (First Kind): Directly measure the activity of metal ions (e.g., Ag, Cu, Zn electrodes).
b. Metal Electrodes (Second Kind): Measure anions that form sparingly soluble salts with metal ions (e.g., Ag electrode for Cl⁻ detection).
c. Redox Electrodes: Used for oxidation-reduction reactions (e.g., platinum electrode for Fe²⁺/Fe³⁺ systems).
d. Ion-Selective Electrodes (ISEs): Most commonly used electrodes for potentiometry. Respond selectively to specific ions. Examples:
- pH Electrode (Glass Electrode) – Measures H⁺ concentration.
- Fluoride Ion-Selective Electrode – Used in fluoride determination.
- Sodium Ion-Selective Electrode – Used in clinical and industrial analysis.
Potentiometric Titrations
Potentiometric titrations involve measuring the potential change during titration instead of using visual indicators. The endpoint is determined from the inflection point in the titration curve.
Types of Potentiometric Titrations
- Acid-Base Titrations
- Used for titrations of strong and weak acids/bases.
- Example: HCl vs. NaOH titration using a pH electrode.
2. Redox (Oxidation-Reduction) Titrations
- Example: KMnO₄ vs. Fe²⁺ using a platinum electrode.
3. Precipitation Titrations
- Used for titrations where a precipitate forms.
- Example: AgNO₃ vs. Cl⁻ using a silver electrode.
- The sharp change in potential corresponds to the precipitation of AgCl.
4. Complexometric Titrations
- Used for titrations involving complex formation.
- Example: EDTA vs. Ca²⁺ using a calcium-selective electrode.
Applications of Potentiometry
1. Pharmaceutical Analysis
- pH determination of drug formulations.
- Purity testing of pharmaceutical compounds.
- Determination of ion concentrations in drug formulations.
2. Environmental Monitoring
- Measurement of pH in water and soil.
- Detection of fluoride and chloride in drinking water.
- Monitoring heavy metal contamination using ion-selective electrodes.
3. Clinical and Biomedical Applications
- Blood and urine pH measurement.
- Determination of electrolyte levels (e.g., Na⁺, K⁺, Ca²⁺) in blood samples.
- Detection of physiological ions for medical diagnosis.
4. Food and Beverage Industry
- Monitoring acidity in beverages (e.g., pH of wine and juices).
- Measurement of salt content in food products.
- Determination of fluoride in toothpaste.
5. Industrial Applications
- pH control in chemical manufacturing.
- Quality control in electroplating industries.
- Monitoring corrosion potential in industrial systems.
Advantages of Potentiometry
- Highly sensitive and selective.
- Can be used for turbid or colored solutions where visual indicators fail.
- It requires minimal sample preparation.
- Non-destructive analysis technique.
- Portable and suitable for field measurements.
Limitations of Potentiometry
- Highly sensitive and selective.
- Can be used for turbid or colored solutions where visual indicators fail.
- It requires minimal sample preparation.
- Non-destructive analysis technique.
- Portable and suitable for field measurements.
Conclusion
Potentiometry is a widely used electroanalytical technique for measuring ion concentrations in various fields, including pharmaceuticals, environmental monitoring, and clinical diagnostics. Its high accuracy, simplicity, and ability to analyze colorless solutions make it an essential tool in modern analytical chemistry. The use of ion-selective electrodes continues to expand, making potentiometry a crucial method in chemical analysis.



