Introduction
Ceric Ammonium Sulfate (CAS), (NH₄)₄Ce(SO₄)₄·2H₂O, is a powerful oxidizing agent widely used in analytical chemistry, particularly in redox titrations. It serves as a reliable volumetric reagent in various quantitative analyses, including the determination of iron, chromium, arsenic, and organic compounds.
CAS is highly stable in acidic solutions but tends to hydrolyze in neutral or basic conditions. To maintain its oxidizing strength, it is typically prepared in the presence of sulfuric acid (H₂SO₄), preventing decomposition and ensuring long-term stability.
The accurate preparation and standardization of CAS solutions are essential for ensuring precise analytical results. Standardization is typically performed using primary standards such as sodium oxalate (Na₂C₂O₄) or arsenic trioxide (As₂O₃), which react in a well-defined stoichiometric manner with ceric ions (Ce⁴⁺).
This guide provides a step-by-step method for the preparation and standardization of CAS molar and normal solutions, ensuring reproducibility, accuracy, and reliability in laboratory experiments. It also discusses the importance of proper storage and handling to preserve the solution’s integrity over time.

Understanding Molarity and Normality of Ceric Ammonium Sulfate
- Molarity (M): Moles of ceric ammonium sulfate per liter of solution.
- Normality (N): Equivalent moles per liter, depending on the reaction.
For oxidation-reduction reactions:
- In an acidic medium, 1M CAS = 1N CAS (as it donates one electron per mole).
Preparation of Ceric Ammonium Sulfate Molar and Normal Solutions
1. Materials Required
- Ceric ammonium sulfate (CAS) crystals
- Distilled water
- Sulfuric acid (H₂SO₄) (1M solution)
- Analytical balance
- Volumetric flask (1L)
- Beaker and glass rod
- Storage bottle (amber-colored)
2. Calculation for Ceric Ammonium Sulfate Solution Preparation
The molar mass of Ceric Ammonium Sulfate Solution is 632.54 g/mol.
Using the formula:
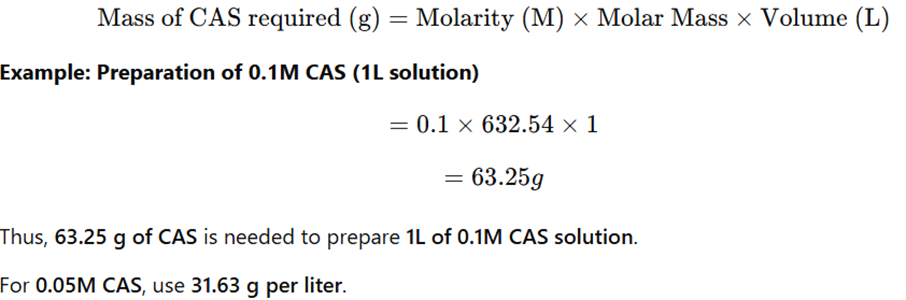
3. Procedure for Preparation
- Weigh the required amount of CAS (e.g., 63.25 g for 0.1M).
- Dissolve in ~500 mL of distilled water in a beaker while stirring continuously.
- Add 10 mL of 1M sulfuric acid (H₂SO₄) to maintain stability and prevent hydrolysis.
- Transfer the solution into a 1L volumetric flask and make up the volume to 1L with distilled water.
- Store in an amber-colored bottle to prevent degradation from light.
⚠ Safety Note: Ceric Ammonium Sulfate Solution is a strong oxidizer-handle with gloves and avoid contact with reducing agents.
Standardization of Ceric Ammonium Sulfate Solution
Since Ceric Ammonium Sulfate Solution degrades over time, it must be standardized using a primary standard, such as arsenic trioxide (As₂O₃) or sodium oxalate (Na₂C₂O₄).
1. Materials Required for Standardization
- Prepared Ceric Ammonium Sulfate Solution
- Arsenic trioxide (As₂O₃) (Primary standard)
- Sulfuric acid (H₂SO₄) (1M solution)
- Starch indicator (optional)
- Burette and pipette
- Conical flask
- Distilled water
2. Reaction Equation
In an acidic medium, ceric ammonium sulfate oxidizes arsenic trioxide:

3. Procedure for Standardization
- Weigh 0.247 g of arsenic trioxide (As₂O₃) and dissolve in 250 mL of distilled water.
- Pipette 25 mL of arsenic trioxide solution into a conical flask.
- Add 10 mL of 1M H₂SO₄ to acidify the solution.
- Titrate with CAS solution from the burette until a color change from yellow to colorless occurs.
- Record the final burette reading and repeat for accuracy.
4. Calculation of Standardized Molarity
Using the titration formula:
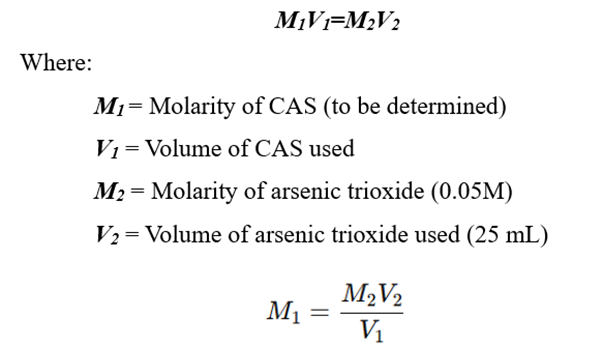
This determines the exact concentration of the Ceric Ammonium Sulfate Solution.
Applications of Standardized Ceric Ammonium Sulfate Solution
- Redox titrations in pharmaceutical and industrial laboratories.
- Determination of iron, chromium, and other reducing agents.
- Oxidation-reduction studies in analytical chemistry.
- Quality control testing in chemical industries.
Conclusion
The preparation and standardization of Ceric Ammonium Sulfate (CAS) molar and normal solutions are vital for precise redox titrations and chemical analysis. Due to ceric ammonium sulfate instability over time, it must be standardized regularly to maintain accurate concentration. Proper storage in dark bottles and acidification with H₂SO₄ ensures solution stability. Additionally, following correct titration techniques and safety measures minimizes errors and enhances experimental accuracy. Regular standardization is recommended to counteract potential degradation.
Mastering these techniques ensures high accuracy in laboratory experiments and industrial applications, making ceric ammonium sulfate a crucial reagent in analytical chemistry.
For more scientific insights and laboratory guides, stay connected!



