Introduction
Hydrochloric acid (HCl) is one of the most commonly used acids in laboratories for various chemical reactions, titrations, and industrial applications. However, proper preparation and standardization of HCl solutions is essential to ensuring accuracy in analytical procedures. This guide will walk you through the step-by-step process of preparing molar and normal solutions of HCl and their standardization.
What is hydrochloric acid (HCl)?
Hydrochloric acid is a strong, highly corrosive acid with the chemical formula HCl. It is widely used in:
- Acid-base titrations
- pH adjustments in laboratories and industries
- Chemical synthesis and industrial processes
- Cleaning and pickling of metals
To ensure consistency and precision, it is important to prepare and standardize HCl solutions before use in analytical procedures.
Preparation of HCl Solutions (Molar and Normal Solutions)

1. Understanding Molarity and Normality of HCl
- Molarity (M): The number of moles of solute (HCl) per liter of solution.
- Normality (N): The number of gram-equivalent weights of solute per liter of solution. Since HCl has a single replaceable hydrogen ion (H⁺), its molarity and normality are the same in aqueous solutions.
For example:
- 1M HCl = 1N HCl
- 0.5M HCl = 0.5N HCl
2. Materials Required
- Concentrated HCl (usually 36-38% w/w, density ~1.18 g/mL)
- Distilled water
- Volumetric flask (1L)
- Measuring cylinder
- Pipette and beaker
- Glass rod for mixing
- Safety equipment (gloves, goggles, lab coat)
3. Calculation for HCl Solution Preparation
Concentrated HCl has a molarity of about 12 M. To prepare a dilute solution, use the dilution formula:

Example: Preparation of 1M HCl (1L solution)
Using the formula:

So, 83.3 mL of concentrated HCl is needed to prepare 1L of 1M HCl solution.
4. Procedure for Preparation
- Add distilled water to a 1L volumetric flask (about 500 mL).
- Slowly add 83.3 mL of concentrated HCl while stirring continuously.
- Once mixed, add more distilled water to make up the volume to 1000 mL (1L).
- Mix well and label the flask appropriately.
⚠ Safety Tip: Always add acid to water, not the other way around, to prevent splashing and heat buildup.
Standardization of Hydrochloric Acid (HCl) Solution
Since concentrated HCl is volatile, its strength may vary, requiring standardization using a primary standard like sodium carbonate (Na₂CO₃).
1. Materials Required for Standardization
- Prepared HCl solution
- Anhydrous sodium carbonate (Na₂CO₃) (Primary standard)
- Methyl orange indicator
- Distilled water
- Burette, pipette, conical flask
2. Reaction Equation

3. Procedure for Standardization
- Weigh 0.53 g of anhydrous Na₂CO₃ and dissolve in 100 mL of distilled water.
- Pipette 25 mL of Na₂CO₃ solution into a conical flask.
- Add 2-3 drops of methyl orange indicator (turns yellow in alkaline solution).
- Fill the burette with HCl solution and note the initial reading.
- Titrate slowly while swirling until the color changes from yellow to orange-red (endpoint).
- Record the final burette reading and calculate the exact molarity of HCl.
4. Calculation of Standardized Molarity
Using the titration formula:

Rearrange to find M₁ (HCl molarity):
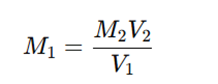
This gives the accurate concentration of the prepared HCl solution.
Applications of Standardized HCl Solution
- Analytical Chemistry: Used in acid-base titrations.
- Pharmaceutical Industry: In quality control and drug formulation.
- Food Industry: For pH adjustments in food processing.
- Water Treatment: Neutralization of alkaline solutions.
Conclusion
The preparation and standardization of hydrochloric acid (HCl) molar and normal solutions are crucial for accurate laboratory experiments. You can ensure precision in chemical analyses and maintain solution consistency by carefully following the steps outlined above. By carefully following the step-by-step procedures outlined above, you can minimize errors, improve reproducibility, and enhance the accuracy of chemical analyses. Additionally, ensuring proper storage and periodic re-standardization of HCl solutions is vital to maintain their strength and effectiveness over time.
For more laboratory guides and scientific insights, stay tuned!
Also read: What is normality?



