Pharmaceutical pastes are semisolid dosage forms containing a high concentration of finely dispersed solids. Unlike ointments, which contain a lower percentage of solids, pastes are stiffer and provide prolonged drug contact with the skin or mucous membrane. They are widely used in dermatology, oral care, and wound management.
Understanding the correct methods of paste preparation ensures product stability, effectiveness, and safety. This guide provides an in-depth analysis of paste formulation, including key ingredients, preparation techniques, and quality control measures.
What Are Pharmaceutical Pastes?
Pharmaceutical pastes are thick, semisolid preparations containing 20–50% finely dispersed solid particles in a base. The presence of solid materials makes pastes less greasy, more absorbent, and suitable for protective and therapeutic applications.
Types of Pastes
Pastes can be categorized based on their function and base type:
1. Medicated Pastes
- Antiseptic Pastes – Contain antimicrobial agents such as zinc oxide or povidone-iodine to prevent infections.
- Anti-inflammatory Pastes – Include corticosteroids (e.g., hydrocortisone) to treat skin inflammation.
- Analgesic Pastes – Contain pain-relieving agents like salicylic acid or menthol.
- Dental Pastes – Used for oral health, often containing fluoride or calcium phosphate.
2. Non-Medicated Pastes
- Protective Pastes – Used as a barrier against irritants (e.g., diaper rash pastes containing zinc oxide).
- Absorbent Pastes – Help absorb exudates from wounds or burns.
Essential Ingredients in Paste Formulation
The effectiveness of a pharmaceutical paste depends on the appropriate combination of active and inactive ingredients.
1. Active Pharmaceutical Ingredients (API)
- API provides the therapeutic effect of the paste.
- Examples: Zinc oxide (for skin protection), fluoride (for dental applications), and salicylic acid (for acne treatment).
2. Base or Vehicle
The base provides the medium for dispersion of the active ingredient. Common bases include:
- Oleaginous Bases – Petrolatum or mineral oil, offering occlusive properties.
- Water-Soluble Bases – Glycerin or polyethylene glycol (PEG), ensuring ease of removal.
- Absorption Bases – Lanolin or beeswax, capable of absorbing water and retaining moisture.
3. Thickening Agents
Thickening agents stabilize the solid content and maintain consistency. Examples include:
- Bentonite
- Starch
- Colloidal silica
4. Preservatives and Stabilizers
- Prevent microbial contamination and degradation.
- Common preservatives: Methylparaben, propylparaben.
- Common stabilizers: Antioxidants like butylated hydroxytoluene (BHT).
5. Humectants
- Maintain moisture balance and prevent drying.
- Examples: Glycerin, sorbitol.
6. Emulsifiers
- Help in maintaining homogeneity of the formulation.
- Examples: Sodium lauryl sulfate, cetyl alcohol.
Methods of Paste Preparation
The choice of method depends on the nature of the ingredients and the intended use.
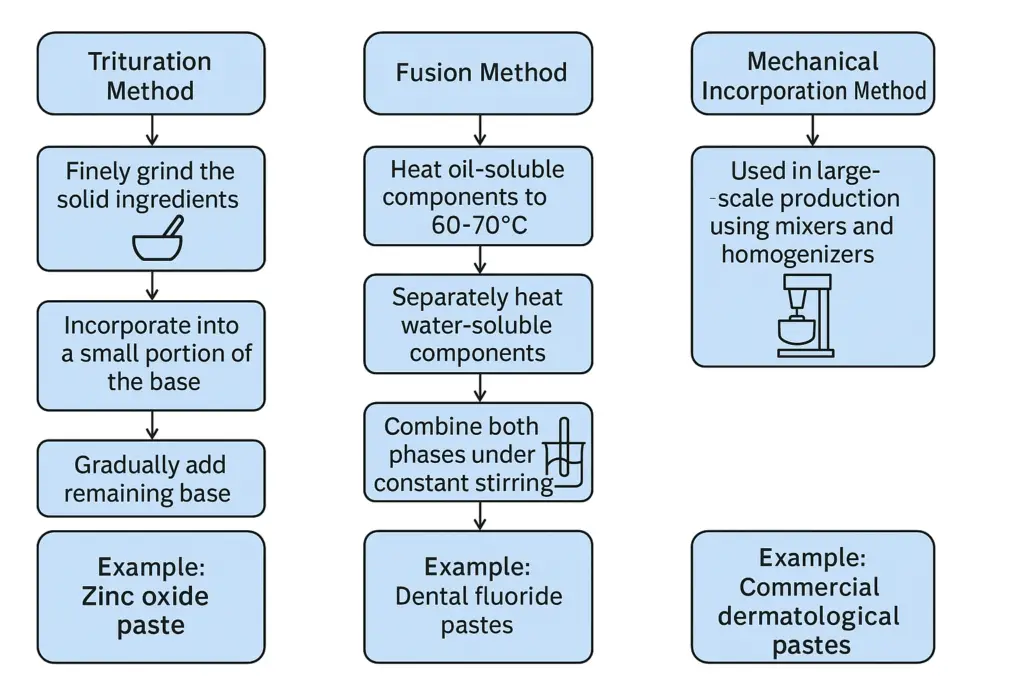
1. Trituration Method
- Used for preparing pastes with insoluble solid particles.
- Steps:
- Finely grind the solid ingredients.
- Incorporate them into a small portion of the base using geometric dilution.
- Gradually add the remaining base to achieve uniform dispersion.
Example: Zinc oxide paste for diaper rash.
2. Fusion Method
- Used when ingredients require heating for uniform blending.
- Steps:
- Heat oil-soluble components to 60-70°C.
- Separately heat water-soluble components.
- Combine both phases under constant stirring.
- Allow to cool and homogenize.
Example: Dental fluoride pastes.
3. Mechanical Incorporation Method
- Used in large-scale production using mixers and homogenizers.
- Ensures even distribution of drug particles and maintains consistency.
Example: Commercial dermatological pastes.
Quality Control in Paste Preparation
Ensuring quality is critical for the safety and effectiveness of pharmaceutical pastes. The following tests are performed:
1. Physical Appearance and Consistency
- It should be smooth, homogeneous, and free from grittiness.
- Evaluated by visual inspection and texture analysis.
2. Spreadability Test
- Measures how easily the paste spreads on the skin or mucosal surface.
- Conducted using a spreadability apparatus.
3. pH Measurement
- Ensures compatibility with skin and mucous membranes.
- Typically, dermatological pastes have a pH close to 5.5.
4. Drug Content Uniformity
- Confirms even distribution of API.
- Measured using high-performance liquid chromatography (HPLC).
5. Stability Testing
- Assesses physical and chemical stability over time.
- Conducted under accelerated conditions (40°C, 75% RH).
6. Microbial Testing
- Ensures product is free from microbial contamination.
- Includes sterility testing and total viable count (TVC) assessment.
7. Viscosity Measurement
- Determines the thickness and flow properties.
- Measured using a viscometer.
Common Issues in Paste Preparation and Solutions
| Issue | Cause | Solution |
| Paste is too thick | Excess solids | Adjust solid-to-base ratio |
| Phase separation | Incomplete mixing | Use appropriate emulsifiers and mix thoroughly |
| Grittiness | Large particle size | Use finer grinding techniques and levigating agents |
| Microbial growth | Poor preservation | Add suitable preservatives |
Advantages of Pastes
- Provide prolonged drug contact.
- Less greasy than ointments.
- Absorb moisture and secretions.
Disadvantages of Pastes
- It can be difficult to spread.
- Require preservatives to prevent microbial growth.
- Some pastes dry out over time.
Common Challenges in Paste Formulation and How to Overcome Them
Formulating pharmaceutical pastes presents several technical challenges that can impact their efficacy, stability, and user experience. Understanding these issues and applying effective solutions ensures consistent quality.
1. Texture Inconsistencies and Phase Separation
One of the most common problems in paste formulation is achieving a uniform texture. Improper mixing, incompatible ingredients, or incorrect ratios of solids to liquids can lead to phase separation, making the paste unstable.
Causes:
- Incorrect particle size distribution of the solid phase
- Inadequate emulsification or thickening agents
- Variability in base components leading to instability
Solutions:
- Optimized Mixing Techniques: Use high-shear mixers or homogenizers to ensure uniform dispersion of solids.
- Selection of Appropriate Thickening Agents: Ingredients like hydrocolloids (e.g., carbomers and cellulose derivatives) help stabilize the formulation.
- Controlled Manufacturing Conditions: Temperature and humidity should be regulated to prevent ingredient instability.
2. Stability and Microbial Contamination Risks
Pharmaceutical pastes contain a high proportion of water or hydrophilic bases, making them more susceptible to microbial contamination and degradation over time. Stability issues can also arise due to oxidation, hydrolysis, or pH imbalance.
Causes:
- Lack of adequate preservatives
- Exposure to moisture and air leading to microbial growth
- Degradation of active ingredients due to environmental factors
Solutions:
- Use of Antimicrobial Preservatives: Ingredients like parabens, benzalkonium chloride, or phenoxyethanol help prevent microbial growth.
- pH Adjustments: Maintain a pH range suitable for both stability and skin compatibility (e.g., pH 4.5–6.5 for dermatological pastes).
- Advanced Packaging: Air-tight and light-resistant packaging minimizes exposure to oxygen and UV radiation, prolonging shelf life.
- Stability Testing: Conduct accelerated and real-time stability testing under different conditions (temperature, humidity) to predict shelf life.
Future Innovations in Paste Formulations
With advancements in pharmaceutical and material sciences, newer paste formulations aim to enhance drug delivery, increase stability, and improve patient compliance.
1. Use of Nanotechnology and Bioadhesive Formulations
Nanotechnology is revolutionizing pharmaceutical pastes by improving drug solubility, penetration, and controlled release.
Key Innovations:
- Nanoparticles and Nanoemulsions: Increase bioavailability of poorly soluble drugs.
- Bioadhesive Polymers: Polymers like chitosan and carbopol enhance the adherence of pastes to mucosal surfaces, improving drug absorption.
- Liposomal Pastes: These allow encapsulation of active drugs in lipid bilayers, facilitating deeper skin penetration.
Applications:
- Wound Healing Pastes: Enhanced penetration of antibacterial and anti-inflammatory agents.
- Oral Care: Bioadhesive dental pastes provide sustained drug release for treating gum infections and sensitivity.
2. Smart Pastes with Controlled Drug Release
Emerging research focuses on “smart pastes” capable of delivering drugs in a controlled manner based on external triggers like temperature, pH, or moisture.
Examples:
- Thermo-responsive pastes: These change viscosity based on body temperature, ensuring better drug absorption.
- pH-Sensitive Pastes: Designed for targeted drug release in different environments (e.g., acidic vs. neutral pH in wounds).
- Hydrogel-Based Pastes: Act as reservoirs for drugs, releasing them gradually over time.
Future Prospects
- Personalized medicine integrating AI-based formulation techniques.
- Development of biodegradable pastes to reduce environmental impact.
Conclusion
Pharmaceutical pastes play an essential role in dermatological and dental treatments. Selecting the right ingredients, using the appropriate preparation method, and ensuring rigorous quality control are key to developing effective and stable formulations. Advancements in nanotechnology and novel excipients continue to enhance paste efficacy and patient compliance.
Frequently Asked Questions (FAQs)
1. What is the difference between a paste and an ointment?
Answer: Pastes contain a higher percentage of solids (20-50%) compared to ointments, making them thicker and less greasy.
2. How do you ensure the stability of a pharmaceutical paste?
Answer: Stability is ensured through proper formulation, the use of stabilizers and preservatives, and conducting stability tests.
3. What are the common bases used in pastes?
Answer: Common bases include petrolatum, lanolin, glycerin, and polyethylene glycol.
4. Why do some pastes dry out over time?
Answer: Loss of moisture due to evaporation. Adding humectants like glycerin can help retain moisture.
5. What tests are performed for paste quality control?
Answer: Tests include drug content uniformity, spreadability, stability, microbial testing, and viscosity measurement.