Introduction
Sodium thiosulfate (Na₂S₂O₃) is widely used in laboratories for iodometric titrations, water treatment, and medical applications. To ensure accurate and reproducible results, the preparation and standardization of sodium thiosulfate molar and normal solutions is crucial. This guide provides a step-by-step approach to preparing and standardizing sodium thiosulfate solutions for laboratory use.
What is Sodium Thiosulfate (Na₂S₂O₃)?
Sodium thiosulfate is a white, crystalline compound that dissolves readily in water. It is commonly used in:
- Iodometric titrations (as a reducing agent)
- Photographic processing (as a fixer)
- Water purification (for chlorine removal)
- Medical treatments (for cyanide poisoning)
Since sodium thiosulfate solutions can decompose over time, proper preparation and standardization are necessary to maintain accuracy in analytical chemistry.

Preparation of Sodium Thiosulfate (Na₂S₂O₃) Molar and Normal Solutions
1. Understanding Molarity and Normality of Na₂S₂O₃
- Molarity (M): The number of moles of Na₂S₂O₃ per liter of solution.
- Normality (N): The number of gram-equivalent weights of Na₂S₂O₃ per liter of solution.
- In redox titrations, sodium thiosulfate acts as a reducing agent, and its normality depends on the reaction equation.
For iodometric titrations, 1M Na₂S₂O₃ = 1N Na₂S₂O₃ since one mole of Na₂S₂O₃ reacts with one mole of iodine (I₂).
2. Materials Required
- Sodium thiosulfate pentahydrate (Na₂S₂O₃·5H₂O)
- Distilled water
- Analytical balance
- Volumetric flask (1L)
- Measuring cylinder
- Glass rod
- Safety equipment (gloves, goggles, lab coat)
3. Calculation for Na₂S₂O₃ Solution Preparation
The molar mass of sodium thiosulfate pentahydrate (Na₂S₂O₃·5H₂O) is 248.18 g/mol.
Example: Preparation of 0.1M Na₂S₂O₃ (1L solution)
Using the formula:
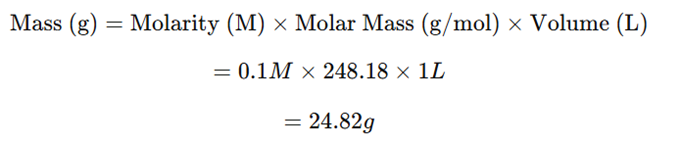
Thus, 24.82 g of sodium thiosulfate pentahydrate is required to prepare 1L of 0.1 M Na₂S₂O₃ solution.
4. Procedure for Preparation
- Weigh the calculated amount (24.82g) of sodium thiosulfate pentahydrate.
- Dissolve it in about 500 mL of distilled water in a volumetric flask.
- Stir gently until completely dissolved.
- Make up the volume to 1L with distilled water.
- Mix well, store in a dark bottle, and label properly.
⚠ Important Note: Sodium thiosulfate decomposes in light and heat, so store the solution in amber-colored bottles at a cool temperature.
Standardization of Sodium Thiosulfate (Na₂S₂O₃) Solution
Since Na₂S₂O₃ solutions can degrade over time, they need to be standardized using a primary standard, typically potassium dichromate (K₂Cr₂O₇) or potassium iodate (KIO₃).
1. Materials Required for Standardization
- Prepared sodium thiosulfate (Na₂S₂O₃) solution
- Potassium iodate (KIO₃) (Primary standard)
- Potassium iodide (KI)
- Sulfuric acid (H₂SO₄)
- Starch indicator
- Distilled water
- Burette, pipette, conical flask
2. Reaction Equation

3. Procedure for Standardization
- Weigh about 0.2g of potassium iodate (KIO₃) and dissolve in 100 mL of distilled water.
- Add excess KI (about 2g) and dilute sulfuric acid (10 mL, 1M) to liberate iodine.
- Titrate the liberated iodine with the prepared Na₂S₂O₃ solution until a pale-yellow color appears.
- Add 1-2 drops of starch indicator, which turns the solution blue-black.
- Continue titration until the blue-black color disappears, indicating the endpoint.
- Record the final burette reading and calculate the exact molarity of Na₂S₂O₃.
4. Calculation of Standardized Molarity
Using the titration formula:
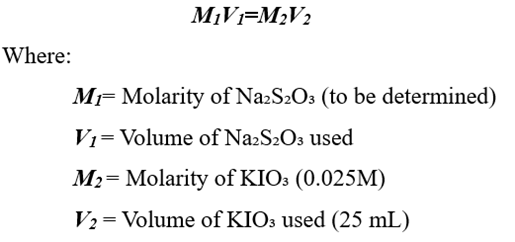
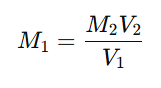
This provides the accurate concentration of the prepared sodium thiosulfate solution.
Applications of Standardized Sodium Thiosulfate Solution
- Iodometric Titrations: Determines oxidizing agents like chlorine, bromine, and iodine.
- Water Treatment: Removes excess chlorine in wastewater.
- Pharmaceutical Analysis: Used in drug formulations and quality control.
- Analytical Chemistry: Employed in redox titrations for chemical assays.
Conclusion
The preparation and standardization of sodium thiosulfate molar and normal solutions are essential for precise and reliable chemical analyses. By following the correct dilution and titration procedures, you can ensure accuracy and reproducibility in iodometric titrations, pharmaceutical quality control, and industrial applications. Since sodium thiosulfate solutions are sensitive to light and temperature, they should be stored in dark, cool conditions and periodically re-standardized to maintain consistent concentration and effectiveness. Mastering these techniques enhances laboratory efficiency and ensures compliance with analytical standards, making sodium thiosulfate a valuable reagent in scientific and industrial chemistry.
For more laboratory insights and analytical guides, stay tuned!
Also read this post: