Redox titrations are a fundamental aspect of volumetric analysis, wherein a solution of known concentration (titrant) reacts with a solution of unknown concentration (analyte) through oxidation and reduction (redox) reactions. These titrations are pivotal in determining the concentration of reducing or oxidizing agents and are widely used in chemical, pharmaceutical, and industrial applications.
or
Redox titrations are those in which we determine the unknown reducing agents using a known amount of oxidizing agent. It is also known as oxidation-reduction titration. Titration involves a redox reaction where oxidation and reduction co-occur.
Concept of Redox Titration
Redox titration is based on the principles of oxidation and reduction reactions, where electron transfer occurs between the titrant and the analyte. for example:
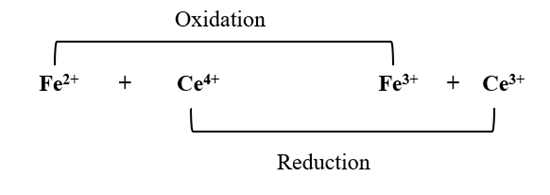
In this reaction, Fe2+ is oxidized and Ce4+ is reduced.
The essential characteristics of these reactions are:
Oxidation Reaction
Oxidation is the loss of electrons by a substance, leading to an increase in its oxidation numbers.
Oxidation involves the following processes:
- Addition of oxygen atoms.
- Removal of hydrogen atoms.
- Loss or donation of electrons.
- Increase in the oxidation state of the substance.
Reduction Reaction
Oxidation is the loss of electrons by a substance, leading to an increase in its oxidation numbers.
Reduction involves the following processes:
- Addition of hydrogen atoms.
- Removal of oxygen atoms.
- Gain of electrons.
- Decrease in the oxidation state of the substance.
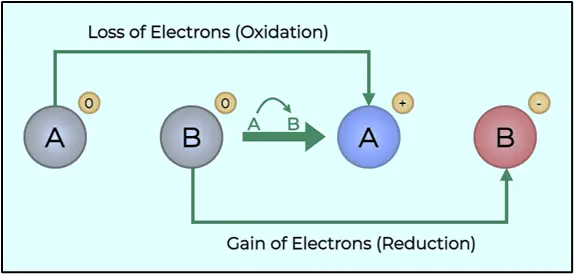
Figure: Concept of Redox Titration
Redox titrations rely on the transfer of electrons between the reacting species. For instance, consider the reaction of iodine solution with a reducing agent. In this process, a starch indicator is used to identify the endpoint of the titration. The diatomic iodine is reduced to iodide ions, resulting in the disappearance of the blue color of the iodine-starch complex. This type of reaction is referred to as iodometric titration.
Principles of Redox Titration
Redox titration is a fundamental analytical technique that relies on oxidation and reduction reactions to quantify substances. Here’s a breakdown of its key principles:
- Oxidation-Reduction Reactions: At the heart of redox titration is the transfer of electrons. One substance undergoes oxidation (losing electrons), while the other undergoes reduction (gaining electrons).
- Titration Endpoint: The endpoint signals the completion of the reaction, often observed as a subtle change in the system, such as a color shift or the formation of a precipitate.
- Equivalence Point: This is the precise moment when the amount of titrant added is stoichiometrically equivalent to the analyte present in the solution, indicating the reaction is fully balanced.
- Titration Curves: Graphs depicting changes in pH or potential during the titration process help visualize the reaction’s progress and pinpoint the equivalence point.
- Choice of Indicator; Selecting the right indicator is crucial; it must exhibit a noticeable color change close to the equivalence point for accurate detection.
- Stoichiometry: A balanced chemical equation guides the calculation of the exact quantity of titrant required to react with the analyte.
- Standardization: To maintain accuracy, the titrant solution is first standardized against a substance with a known concentration before being used in the titration.
In conclusion, redox titrations are a vital analytical tool for quantifying oxidizing and reducing agents through oxidation-reduction reactions. Their precision and versatility make them indispensable in chemical, pharmaceutical, and industrial applications.



