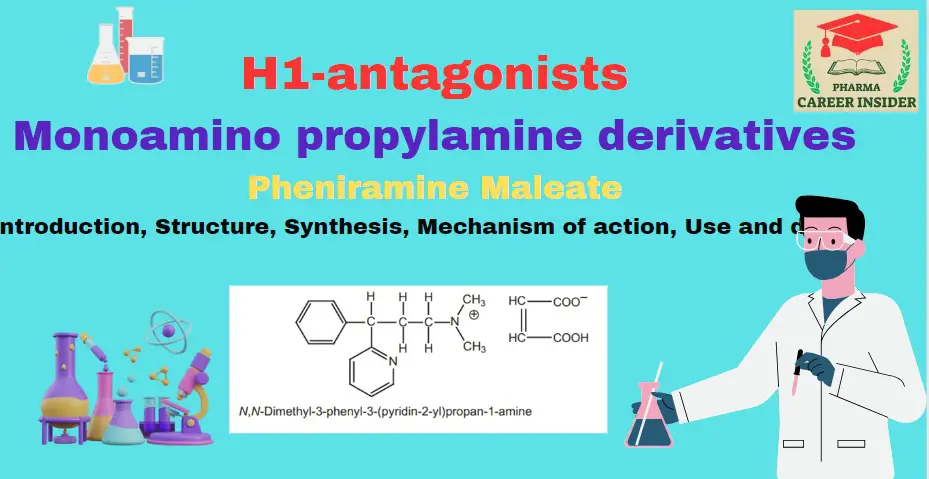
Introduction
A reference electrode is an essential component in electrochemical measurements, providing a stable and well-defined potential against which the potential of other electrodes can be measured. These electrodes do not participate in the reaction but serve as a reference point to compare the working electrode’s potential. The three most commonly used reference electrodes are:
- Standard Hydrogen Electrode (SHE)
- Silver-Silver Chloride Electrode (Ag/AgCl Electrode)
- Saturated Calomel Electrode (SCE)
Each of these electrodes has specific construction, working principles, advantages, and limitations.
Standard Hydrogen Electrode (SHE)
Construction
The Standard Hydrogen Electrode (SHE) is the primary reference electrode with a standard potential of 0.000 V. Its construction includes:
- Platinum Electrode: A thin platinum (Pt) wire or foil coated with platinum black to increase surface area and facilitate hydrogen ion exchange.
- Hydrogen Gas Supply: Pure hydrogen gas (H₂) is continuously bubbled over the platinum electrode at a pressure of 1 atm.
- Acidic Solution: The electrode is immersed in a 1M strong acid solution (commonly HCl or H₂SO₄) to provide H⁺ ions.
- Salt Bridge: A salt bridge is used to maintain electrical neutrality and complete the electrochemical circuit.

Working
The SHE operates based on the hydrogen redox reaction:

- The platinum electrode serves as a medium for electron transfer.
- Hydrogen gas bubbles over the electrode, maintaining equilibrium with the H⁺ ions in solution.
- The potential of any unknown electrode is measured against SHE using a potentiometer.
- Since its potential is always taken as 0.000V, SHE serves as a universal standard for all electrochemical measurements.
Advantages
- Provides a universal reference potential (0V).
- Highly accurate and reproducible.
Disadvantages
- Requires a continuous supply of pure hydrogen gas, making it impractical for routine use.
- Platinum electrodes can be poisoned by impurities, affecting their stability.
- Difficult to handle and maintain in laboratory conditions.
Silver-Silver Chloride Electrode (Ag/AgCl Electrode)
Construction
The Silver-Silver Chloride Electrode (Ag/AgCl) is a widely used reference electrode due to its simplicity and stability. It consists of:
- Silver Wire (Ag): A silver electrode is coated with a layer of silver chloride (AgCl).
- KCl Solution: The electrode is immersed in a potassium chloride (KCl) solution of known concentration (commonly saturated KCl or 1M KCl).
- Porous Junction: A porous frit or salt bridge allows ionic contact with the test solution without direct mixing.
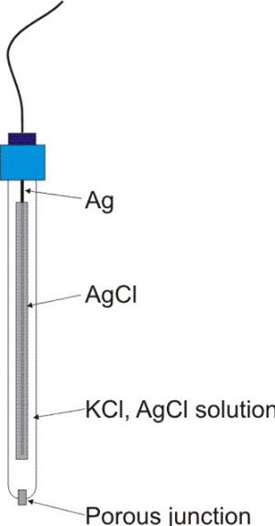
Figure: Silver-Silver Chloride Electrode (Ag/AgCl Electrode)
Working
The Ag/AgCl electrode functions based on the following half-cell reaction:

- AgCl undergoes a reversible reaction with silver metal (Ag) and chloride ions (Cl⁻) in solution.
- The electrode potential depends on the Cl⁻ concentration, which determines the stability of the reference potential.
- The potential values for different KCl concentrations are:
Saturated KCl: E∘ = +0.197VE vs SHE
1M KCl: E∘ = +0.222V vs SHE
Advantages
✖ Potential is dependent on Cl⁻ concentration, requiring strict control over solution conditions.
✖ Not as stable over long-term use as SCE.
Disadvantages
✖ Potential is dependent on Cl⁻ concentration, requiring strict control over solution conditions.
✖ Not as stable over long-term use as SCE.
Saturated Calomel Electrode (SCE)
Construction
The Saturated Calomel Electrode (SCE) is another commonly used reference electrode, based on mercury and calomel (Hg₂Cl₂). It consists of:
- Mercury (Hg): A pool of liquid mercury acts as the conductive electrode.
- Calomel (Hg₂Cl₂): Mercury(I) chloride (calomel) is used to maintain a stable reaction equilibrium.
- KCl Solution: The electrode is placed in a saturated potassium chloride (KCl) solution, ensuring a constant Cl⁻ concentration.
- Porous Junction: A porous frit allows ionic exchange with the test solution.
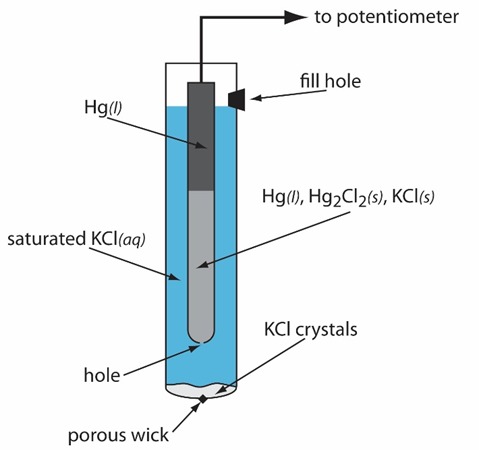
Working
The SCE electrode works based on the redox reaction:

- The calomel (Hg₂Cl₂) maintains equilibrium with mercury (Hg) and chloride ions (Cl⁻).
- The electrode potential varies with the concentration of KCl:
Saturated KCl: E∘ = +0.241VE vs SHE
1M KCl: E∘ = +0.280V vs SHE
- The SCE is more stable than the Ag/AgCl electrode and is widely used in laboratory applications.
Advantages
- Provides stable and well-defined potential.
- Less sensitive to temperature variations.
- More reliable than the SHE in practical applications.
Disadvantages
- The toxicity of mercury makes it environmentally hazardous.
- Can leak KCl solution, contaminating the system.
Comparison of Reference Electrodes

Conclusion
Reference electrodes play a crucial role in electrochemical measurements by providing a stable and known potential against which other electrodes are compared. The Standard Hydrogen Electrode (SHE) serves as the universal reference but is impractical for routine use. The Silver-Silver Chloride (Ag/AgCl) Electrode is widely used due to its simplicity and reliability, while the Saturated Calomel Electrode (SCE) is preferred for its stability but is being replaced due to environmental concerns related to mercury toxicity. Understanding the construction and working principles of these electrodes is essential for their proper application in electrochemical experiments.