Introduction:
Sachse and Mohr (1918) argued that cycloalkanes beyond cyclopentane could be stable if all the ring carbons were not constrained to lie in a single plane, as previously believed by Baeyer. This would result in a ring structure that is free of strain and, thus, more stable. Sachse-Mohr’s “Strainless Rings” concept is a theoretical framework in organic chemistry that suggests that certain cyclic compounds are either free of strain or have minimal strain despite having a cyclic structure. This concept contradicts the traditional belief that all cyclic compounds experience some strain due to deviations from ideal bond angles and lengths.
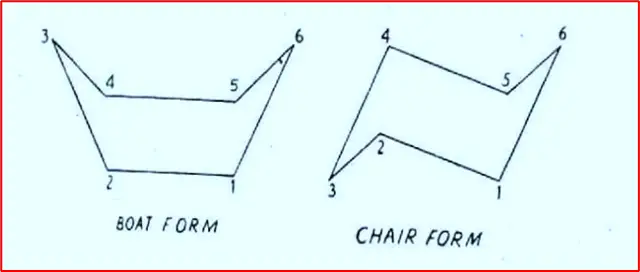
Cyclohexane is a molecule that can exist in two non-planar forms: the Boat and Chair forms. In both forms, the ideal normal tetrahedral angles of 109°28 are retained, which helps relieve the strain within the ring. In the Boat form, carbons 1, 2, 4 and 5 lie in the same plane, while carbons 3 and 6 are above the plane. On the other hand, in the Chair form, carbons 1, 2, 4 and 5 also lie in the same plane, but carbon 6 is above the plane while carbon 3 is below it.
The concept of strainless rings has important implications for the design and synthesis of cyclic organic compounds, as it suggests that certain ring sizes and structural features may confer greater stability and lower reactivity than others. Understanding the principles underlying strainless rings can aid chemists in predicting the stability and behaviour of cyclic molecules and designing more efficient synthetic routes to these compounds.
Limitations of Sachse Mohr’s Concept of Strainless Rings:
Here are the limitations of Sachse Mohr’s Concept of Strainless Rings listed point-wise:
1. Oversimplified Criterion: The concept relies solely on the size of the ring to determine strain, neglecting other contributing factors like steric hindrance and bond angle distortions.
2. Ignoring Steric Effects: It does not consider the potential strain caused by bulky substituents attached to the ring, which can significantly affect the molecule’s stability.
3. Lack of Conformational Analysis: The concept does not account for the possibility of non-planar conformations in larger rings, which can lead to additional strain.
4. Limited Applicability: While it may provide a rough approximation for some cycloalkanes, it fails to fully explain the stability of all cyclic compounds, especially those with complex substituent patterns or unusual ring sizes.
5. Inadequate for Modern Organic Chemistry: With advancements in computational chemistry and conformational analysis, the simplistic approach of Sachse Mohr’s Concept is insufficient to describe cycloalkanes’ stability in detail.




