Stereochemistry involves the study of the three-dimensional nature of molecules. It is the study of the chiral molecules.
- Stereochemistry plays a major role in the pharmacological properties because any change in the stereospecificity of the drug will affect its pharmacological activity.
- The isomeric pairs have different physical properties (log p, pKa, etc.) and thus differ in pharmacological activity.
- The isomers with the same bond connectivity but different arrangements of groups or atoms in space are termed stereoisomers.
Conformational Isomers:
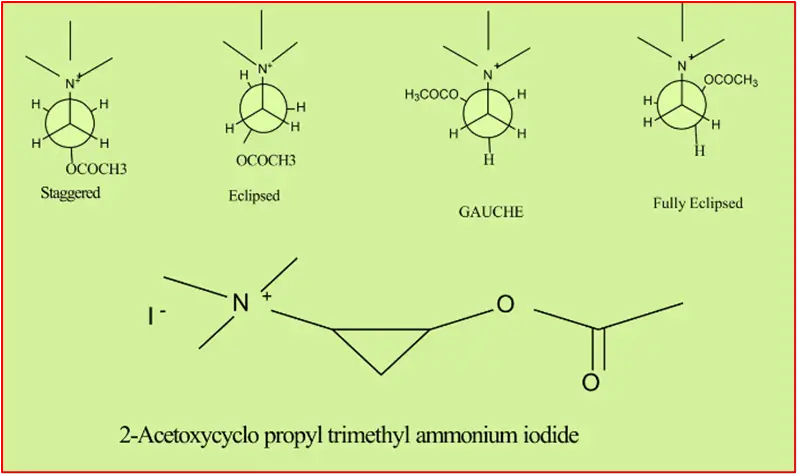
Different arrangements of atoms that can be converted into one another by rotation about single bonds are called conformations. Rotation about bonds allows the inter-conversion of conformers. A classic example is acetylcholine, which can exist in different Conformations.
Optical Isomers:
Optical isomers, also known as enantiomers, are compounds with the same number and type of atoms and bonds, but different spatial arrangements of the atoms. Optical isomers are important in understanding how drugs work and their potential side effects. When a compound contains one asymmetric center, it has two enantiomers. Even though these enantiomers have the same chemical and physical properties, they can behave differently in the body. This is because they interact differently with receptors, undergo metabolic processes, and bind to proteins differently. The reason for this difference in biological activity is that one enantiomer can fit into a receptor molecule with three points of attachment, while its mirror image enantiomer can only fit with two points of attachment.
Optical isomerism arises due to differences in the rotation of plane-polarized light by the drug molecule, i.e., the drug may be (+) dextrorotatory or (-) levorotatory. Various examples are-
There both enantiomers (+) and (-) have different biological activities.
- (-) adrenaline in more active then (+) adrenaline.
- (-) Warfarin is 5 times more potent than (+) isomer.
- (-) Propranolol is more potent than (+) propranolol.
- (+) Amphetamine is 3-4 times more active than (-) isomer.
In certain cases, one isomer is active while the other isomer is toxic. For example-
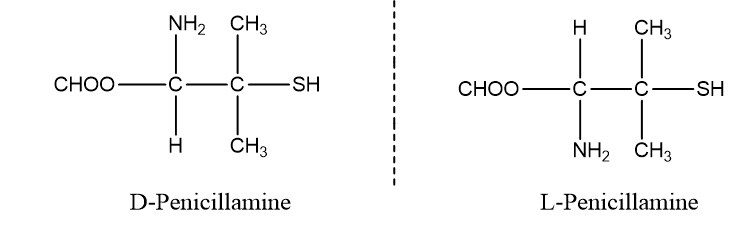
D-Penicillamine is used in the treatment of arthritis while L-Penicillamine is highly toxic.
Geometrical Isomerism:
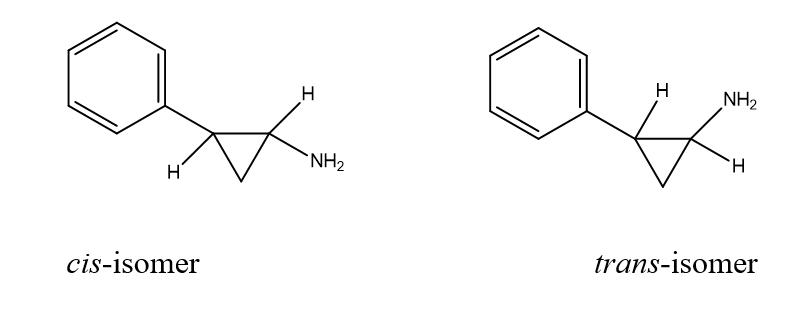
This isomerism is represented by cis and tans isomerism. The isomerism or arrangements of atoms around the C=C bond. It occurs as a result of or due to the presence of (C=C).
For example-
- Trans-diethyl stilbestrol is more potent than Cis-isomer, i.e., higher estrogenic activity.
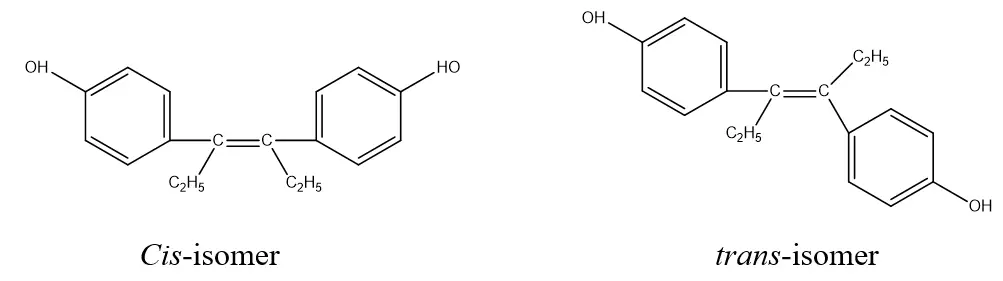
Cis-isomer trans-isomer
- Cis-2-phenylcyclopropylamine is more active as an inhibitor of Monoamine oxides (MAO) than its trans-isomer.
cis-isomer trans-isomer