The structure-activity relationship (SAR) for H1 receptor antagonists, commonly known as antihistamines, involves understanding how the chemical structure of a compound influences its pharmacological activity in blocking the H1 receptors. Here are some key aspects of the SAR for H1 receptor antagonists:
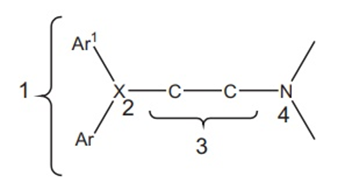
1. Aryl groups
The diaryl substitution is essential for significant H1 affinity. It is present both in first generation and second-generation antihistamines. The optimal antihistaminic activity depends on the co-planarity of two aryl substitutions.
Active aryl substitutions are as follows:
Ar is phenyl and hetero aryl group like 2-pyridyl
Ar1-Aryl or aryl methyl group
2. Nature of X
Antihistamines with X = carbon (pheniramine series) represent the stereo-selective receptor binding to the receptors due to its chirality.
The active substitutions of X are as follows:
Where X = oxygen (aminoalkyl ether analog)
X = nitrogen (ethylene-diamine derivative)
X = carbon (mono amino propyl analogue)
2. The Alkyl Chain
Most of the antihistamines have an ethylene chain, and branching of this chain results in a less active compound. All antihistamines contain this general chain.
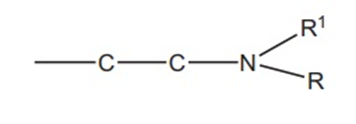
3. Terminal nitrogen atom:
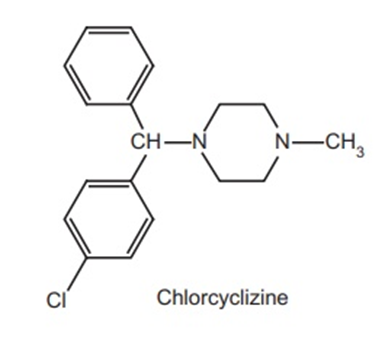
The terminal N-atom should be a 3° amine for maximum activity. The terminal nitrogen may be a part of a heterocyclic ring. For example, antazoline and chlorcyclizine retain high antihistaminic activity. The amino moiety deserves the protonation on interaction with the H1 receptor due to the basicity with pka 8.5-10.