Suppositories are solid dosage forms intended for insertion into body cavities like the rectum, vagina, or urethra. They deliver systemic or localized effects and are widely used for conditions such as pain relief, hemorrhoids, infections, and fever. The choice of preparation method depends on factors like drug stability, base compatibility, and required dosage accuracy.
This article explores four primary methods for suppository preparation and their procedures, advantages, and limitations.
Suppository Preparation Methods
Suppositories are solid dosage forms designed for rectal, vaginal, or urethral administration, formulated using different methods to optimize drug delivery. The molding (fusion) method involves melting the base, mixing in the drug, and pouring it into molds for uniform distribution. The compression method is ideal for heat-sensitive drugs, using high pressure instead of heat. Hand-rolling is a traditional, cost-effective approach for small-scale preparation. Cold compression molding ensures high drug stability without heat exposure. Choosing the right preparation method is essential for ensuring effective drug release, absorption, and patient compliance.
1. Molding (Fusion) Method
Principle:
The molding method, also known as the fusion method, involves melting the suppository base, incorporating the drug, and pouring it into molds.
Procedure:
- Selection of Base: Choose a suitable base like cocoa butter, polyethylene glycol (PEG), or glycerogelatin.
- Melting the Base: Heat the base in a water bath to avoid direct heating.
- Incorporation of Drugs: Add finely powdered active pharmaceutical ingredients (APIs) with continuous stirring.
- Pouring into Molds: Transfer the molten mixture into pre-lubricated suppository molds.
- Cooling and Solidification: Allow the suppositories to cool at room temperature or in a refrigerator.
- Removal from Molds: Gently remove the hardened suppositories from the molds and package them.
Advantages:
- Suitable for thermolabile and insoluble drugs
- Provides uniform drug distribution
- deal for large-scale production
Disadvantages:
- Risk of sedimentation of drugs in the mold
- Some bases, like cocoa butter require precise temperature control to prevent polymorphic changes
2. Compression Method
Principle:
This method involves compressing a mixture of drugs and a suppository base into molds under high pressure.
Procedure:
- Preparation of Suppository Mass: Mix the drug with a semi-solid base (e.g., hydrogenated fat).
- Filling the Compression Machine: Load the mass into a suppository compression machine.
- Compression: Apply pressure to mold the suppositories into the required shape.
- Ejection and Packaging: Remove the finished suppositories and store them appropriately.
Advantages:
- No heat exposure, making it ideal for heat-sensitive drugs
- Ensures accurate dosage and weight uniformity
- Reduces risk of sedimentation
Disadvantages:
- Specialized equipment is required, making it costly for small-scale production
- Limited to certain types of suppository bases
3. Hand-Rolling Method
Principle:
The oldest and simplest method, where drug and base are kneaded together and shaped manually.
Procedure:
- Grinding the Drug: Convert the API into a fine powder.
- Mixing with Base: Knead the powder with a suppository base (e.g., cocoa butter) to form a uniform mass.
- Hand-Shaping: Roll the mass into a cylindrical shape and cut it into uniform pieces.
- Smoothing and Packaging: Shape each piece into a tapered end for easy insertion and package them.
Advantages:
- No need for molds or machines
- Simple and cost-effective
- Ideal for small-scale or personalized formulations
Disadvantages:
- Labor-intensive and time-consuming
- High risk of dose variation
- Limited to bases that soften at body temperature, like cocoa butter
4. Pouring into Molds (Cold Compression)
Principle:
A modified method of molding where high-pressure cold compression is used instead of heat.
Procedure:
- Preparation of the Base: Select a solid suppository base like PEG.
- Mixing with Drug: Blend the drug uniformly with the base at room temperature.
- Filling Molds: Pour the mass into molds.
- Compression and Removal: Apply slight pressure for uniformity and remove after solidification.
Advantages:
- Suitable for heat-sensitive drugs
- No risk of polymorphic changes in the base
- Maintains high drug stability
Disadvantages:
- Requires specialized molds
- Limited to specific types of bases
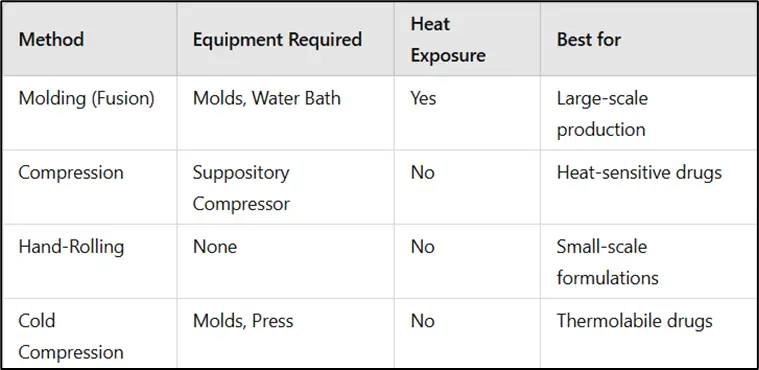
Comparison of Suppository Preparation Methods
Quality Control and Testing of Suppositories
Quality control ensures that suppositories meet pharmaceutical standards for efficacy, stability, and safety. The following tests are commonly performed:
a) Weight Variation
- Ensures consistency in drug content and uniformity across batches.
- Weigh 20 suppositories individually and compare the average weight to the standard limit.
- Variations should be within permissible pharmaceutical limits (±5% for individual weight).
b) Disintegration and Dissolution Tests
- Disintegration Test: Determines how quickly the suppository breaks down in physiological conditions.
- The suppository is placed in a medium at 37°C to mimic body temperature.
- It must disintegrate within the specified time limit (usually ≤30 minutes for rectal and ≤60 minutes for vaginal suppositories).
- Dissolution Test: Measures the rate and extent of drug release.
- Performed using a USP dissolution apparatus with simulated body fluids.
- Helps in evaluating bioavailability and ensuring drug efficacy.
c) Drug Content Uniformity
- Ensures even distribution of the active pharmaceutical ingredient (API).
- A minimum of 10 suppositories are randomly selected and analyzed for drug content using spectrophotometry or chromatography (HPLC).
- Each suppository should contain 85–115% of the labeled drug amount.
Applications of Suppositories
- Pain Management: Rectal suppositories with NSAIDs for pain relief
- Hormonal Therapy: Vaginal suppositories for estrogen delivery
- Fever Reduction: Pediatric rectal suppositories for antipyretics like paracetamol
- Local Infections: Antifungal suppositories for vaginal infections
Factors Affecting Suppository Stability and Shelf Life
The stability and shelf life of suppositories depend on several factors that influence their physical and chemical integrity.
a) Temperature and Storage Conditions
- Fatty bases (e.g., cocoa butter) are prone to melting at high temperatures (>30°C), requiring refrigeration.
- PEG-based suppositories are more stable at room temperature but may absorb moisture.
- Proper packaging (e.g., aluminum foil, PVC blisters) prevents moisture and air degradation.
b) Base Compatibility with APIs
- Drug solubility in the base effects release rate and stability.
- Incompatibility Issues:
- Cocoa butter may undergo polymorphic transitions, leading to variations in melting behavior.
- PEG bases may interact with certain APIs, affecting drug release.
- Compatibility studies using DSC (Differential Scanning Calorimetry) and FTIR (Fourier Transform Infrared Spectroscopy) help identify stable formulations.
Innovations in Suppository Manufacturing
Advancements in pharmaceutical technology are improving suppository formulation for better patient compliance and drug absorption.
a) Novel Excipients for Better Absorption
- Mucoadhesive Polymers: Prolong retention time in rectal/vaginal mucosa for sustained release (e.g., carbopol, HPMC).
- Self-Emulsifying Drug Delivery Systems (SEDDS): Enhances bioavailability of poorly soluble drugs.
- Nanoformulations: Lipid nanoparticles improve drug solubility and absorption in suppositories.
b) Advanced Molding Techniques
- 3D Printing of Suppositories: Allows precise drug dosing and customization.
- Microwave-Assisted Fusion Technology: Reduces preparation time and enhances uniformity.
- Cryogenic Molding: Maintains API stability during manufacturing by preventing thermal degradation.
Conclusion
Choosing the right suppository preparation method is crucial for ensuring stability, drug release, and therapeutic efficacy. Molding, compression, hand-rolling, and cold compression techniques each have unique advantages depending on drug characteristics and production requirements. Proper selection and preparation enhance patient compliance and treatment success.
Frequently Asked Questions (FAQs)
1. Which method is best for preparing heat-sensitive drugs?
The compression method and cold compression are best as they do not involve heat exposure.
2. Why is cocoa butter a preferred base for suppositories?
Cocoa butter melts at body temperature, ensuring quick drug release and ease of administration.
3. Can all drugs be incorporated into suppositories?
No, only drugs that remain stable in lipophilic or hydrophilic bases and require local or systemic absorption are suitable.
4. Which suppository preparation method is most commonly used in the pharmaceutical industry?
The molding (fusion) method is widely used for large-scale production due to its efficiency and uniformity.
5. How are suppositories stored?
Suppositories should be stored in a cool, dry place (refrigerated if necessary) to prevent melting or degradation. If you found this article useful, share it with others! Let us know your thoughts in the comments below.



