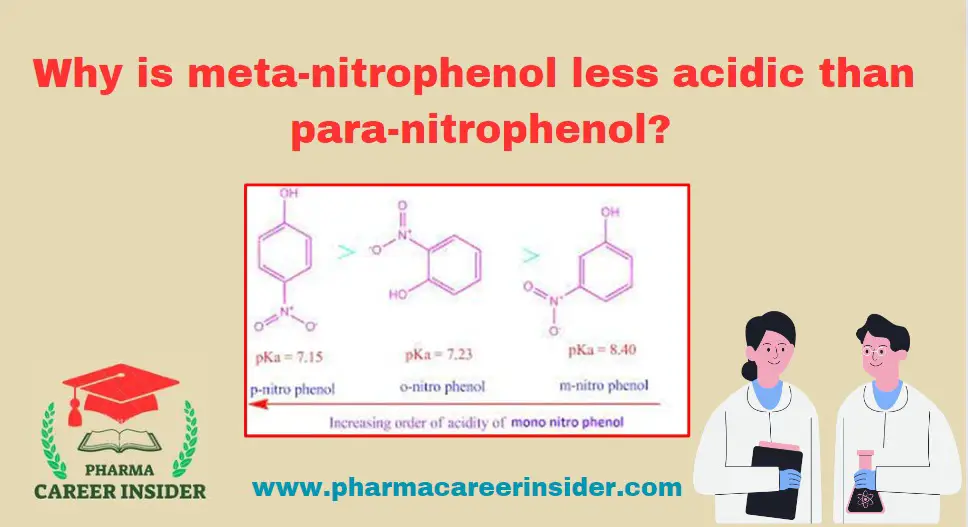
Why is meta-nitrophenol less acidic than para-nitrophenol?
The position of the substituent on the aromatic ring influences the acidity of substituted phenols. In the case of meta-nitrophenol (m-nitrophenol) and para-nitrophenol (p-nitrophenol), the …