Introduction
An acid-base titration is a quantitative analytical method used to determine the concentration of an unknown acid or base by neutralizing it with a solution of known concentration. The process relies on the fundamental principles of acid-base reactions and involves different theoretical models to explain their behavior. The titration curve, which represents the pH change during titration, varies depending on the strength of the acid and base involved. Theories such as Arrhenius, Brønsted-Lowry, and Lewis provide a scientific basis for understanding acid-base interactions, while concepts like the equivalence point and indicators play a critical role in detecting the completion of the reaction. Acid-base titrations are widely used in industries, pharmaceuticals, and research laboratories for precise chemical analysis.
Several models have been developed to classify, rationalize, and predict the reactivity of acid-base pairs (or donor-acceptor pairs). These include:
- Arrhenius Model (Water Theory): Acids produce hydrogen ions (H⁺) in aqueous solutions, while bases produce hydroxide ions (OH⁻) in aqueous solutions.
- Brønsted-Lowry Model (Proton Theory): Acids are proton (H⁺) donors, and bases are proton acceptors.
- Lewis Model (Electronic Theory): Acids are electron pair acceptors, and bases are electron pair donors.
- Electrophile-Nucleophile Model: Acids act as electrophilic reagents, while bases act as nucleophilic reagents.
1. Arrhenius Theory
The Swedish chemist Svante Arrhenius (1887) proposed the first acid-base model, defining acids and bases based on their behavior in water.
Arrhenius Acids and Bases
- Acid: A substance dissociating in water to produce hydrogen ions (H⁺).

- Base: A substance dissociating in water to produce hydroxide ions (OH⁻).
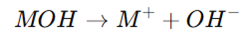
- Neutralization Reaction: Acids and bases react to form water and salt.
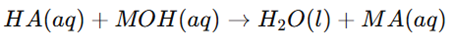
Advantages
- Simple and useful for aqueous solutions.
- Explains acid-base properties of common substances like HCl and NaOH.
Disadvantages
- It cannot explain acid-base reactions in non-aqueous media.
- Does not account for amphoteric substances (e.g., Na₂HPO₄, NaHCO₃).
2. Brønsted-Lowry Theory
Johannes Brønsted and Thomas Lowry decisively expanded Arrhenius’s theory by defining acids and bases based on the transfer of protons, a framework that operates independently of the solvent. This theory is also called proton theory.
Brønsted-Lowry Acids and Bases
- Acid: A substance that donates a proton (H⁺).
- Base: A substance that accepts a proton (H⁺).
Acid-Base Reactions & Conjugate Pairs
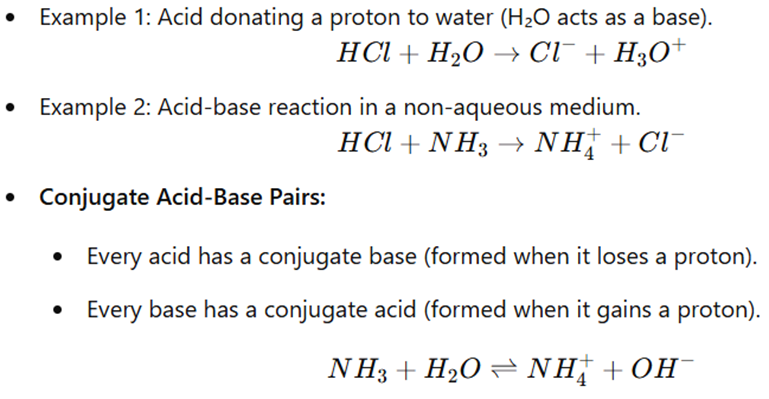
Advantages
- Explain amphiprotic substances (e.g., water can act as both acid and base).
- Independent of solvent type.
Disadvantages
- Does not explain acid-base behavior in non-protonic solvents (e.g., BF₃, POCl₃, SO₂).
3. Lewis Theory
G.N. Lewis introduced this theory in 1923, presenting a broader concept of acids and bases that extends beyond just proton transfer. This theory is also called Electronic Theory.
Lewis Acids and Bases
- Lewis Acid: An electron pair acceptor.
- Examples: BF₃, AlCl₃, Cu²⁺, Fe³⁺, H⁺, SO₃
- Lewis Base: An electron-pair donor.
- Examples: NH₃, OH⁻, H₂O, Cl⁻, O²⁻
Lewis Acid-Base Reactions
- Formation of an adduct (a complex formed by sharing an electron pair).
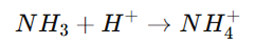
Advantages
- Explains many organic and inorganic reactions.
- The first theory to include non-hydrogen-containing acids like BF₃.
Disadvantages
- Limited to electron-pair donor compounds (must contain O, N, or S).
4. Usanovich Theory
M. Usanovich expanded the Lewis concept (in the year 1934) to include oxidation-reduction reactions. It’s also called the Generalized Acid-Base Concept.
Usanovich Acids and Bases
- Acid: A substance that donates cations, accepts anions, or accepts electrons.
- Base: A substance that donates anions, donates electrons, or reacts with cations.
Examples of Usanovich Acid-Base Reactions
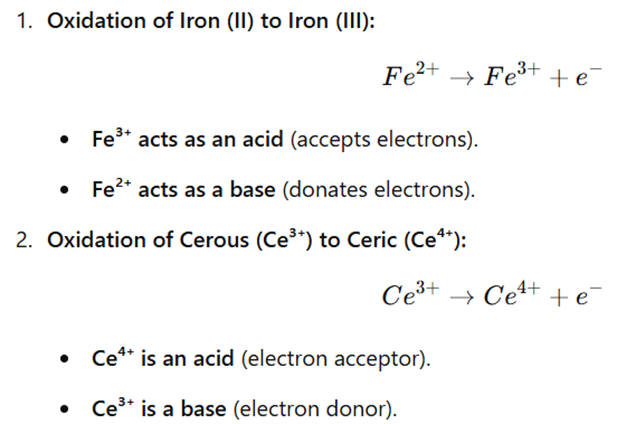
Advantages
- Unifies acid-base and redox reactions into a single framework.
- Broader than Arrhenius, Brønsted-Lowry, and Lewis’s theories.
Disadvantages
- Too broad for practical classification of acid-base reactions.
Conclusion
Each acid-base theory has significantly advanced our understanding of chemical reactivity. Arrhenius’s Theory effectively explains acid-base behavior in aqueous solutions but is limited to water as the solvent. Brønsted-Lowry Theory expands on this by introducing proton transfer, allowing acid-base reactions to be understood beyond aqueous environments. Lewis’s Theory further broadens the definition by focusing on electron pair interactions, providing insights into a wider range of chemical reactions. Usanovich’s Theory generalizes the concept to include redox reactions, though its broad scope makes it less practical for specific applications. These theories collectively enable chemists to analyze reaction mechanisms, predict product formation, and apply acid-base principles in diverse fields, including industrial chemistry and biochemistry. These theories help chemists understand reaction mechanisms, predict product formation, and apply acid-base principles across various fields, from industrial chemistry to biochemistry.