Thin Layer Chromatography (TLC) is a simple, quick, and cost-effective method used for separating and identifying compounds in a mixture. It is widely used in laboratories for qualitative analysis, especially in the fields of chemistry, biochemistry, and pharmacy. TLC helps to determine the purity of substances and monitor the progress of reactions.
Principle of TLC
TLC operates on the principle of adsorption chromatography. A thin layer of a stationary phase (usually silica gel or alumina) is applied to a glass, metal, or plastic plate. A small sample is spotted near the bottom of the plate, and the plate is placed in a solvent (mobile phase). As the solvent moves up the plate through capillary action, the sample components move at different rates depending on their affinities for the stationary phase and their solubility in the mobile phase. This results in the separation of the components.
Methodology (Steps Involved in TLC)
1. Preparation of TLC Plate:
– A thin layer of adsorbent material (silica gel or alumina) is spread on a glass, metal, or plastic plate.
– The plate is activated by drying it in an oven to remove any moisture.
2. Sample Application:
– A small amount of the sample mixture is spotted near the bottom of the TLC plate using a capillary tube or micropipette.
– The spots should be small to prevent overlapping of separated components.
3. Development of the Plate:
– The TLC plate is placed vertically in a chamber containing a small amount of the solvent (mobile phase). The solvent level should be below the sample spots.
– The chamber is covered to saturate the air with the solvent vapor.
– As the solvent moves up the plate by capillary action, it carries the sample components along, separating them based on their interaction with the stationary phase.
4. Drying the Plate:
– Once the solvent has nearly reached the top, the plate is removed from the chamber and allowed to dry.
5. Visualization of the Spots:
– The separated components are visualized by exposing the plate to ultraviolet (UV) light or by spraying it with specific reagents that produce colored spots.
6. Calculation of Rf Values:
– The Rf (retention factor) value of each component is calculated using the formula:
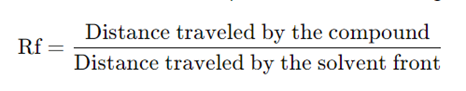
Rf Values
– Rf value is a crucial parameter in TLC. It represents the relative distance traveled by a compound compared to the solvent front. Each compound has a characteristic Rf value in a particular solvent, which helps in its identification.
– Rf value range: It is always between 0 and 1.
– Rf = 0: The compound did not move and is highly attracted to the stationary phase.
– Rf = 1: The compound moved with the solvent and has less affinity for the stationary phase.
Advantages of TLC
– Simple and quick: Requires minimal equipment and setup.
– Cost-effective: Inexpensive method compared to other chromatographic techniques.
– Wide applicability: Can be used for various types of samples, including solids, liquids, and gases.
– Visual observation: The results can be easily seen, especially under UV light.
– Multiple samples: Several samples can be analyzed simultaneously on the same plate.
Disadvantages of TLC
– Lower accuracy: Not as accurate or precise as high-performance techniques like HPLC.
– Limited sensitivity: Not ideal for detecting very low concentrations of compounds.
– Open system: Environmental factors like humidity can affect the separation process.
– Semi-quantitative: Provides mainly qualitative data; quantitative results are less reliable.
Applications of TLC
– Purity check: To determine the purity of pharmaceutical compounds.
– Drug identification: Helps in identifying active ingredients in drug formulations.
– Monitoring reactions: Used to track the progress of chemical reactions.
– Separation of mixtures: Effective for separating and identifying components in a mixture, like plant extracts or synthetic chemicals.
– Biochemical analysis: Used for analyzing amino acids, lipids, and nucleotides.
– Forensic science: Assists in the analysis of drugs, explosives, and other substances in crime investigations.




