Ureido penicillins are a class of antibiotics that are structurally related to penicillin, distinguished by the presence of a ureido group in their chemical structure. This group includes drugs like piperacillin and mezlocillin, which possess a broader spectrum of activity than traditional penicillins, particularly against Gram-negative bacteria. Ureido penicillins inhibit bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs), ultimately leading to cell lysis and death. Their extended spectrum makes them valuable in treating severe infections, including those caused by Pseudomonas aeruginosa and other resistant organisms.
1. Aziocillin (Azlin)
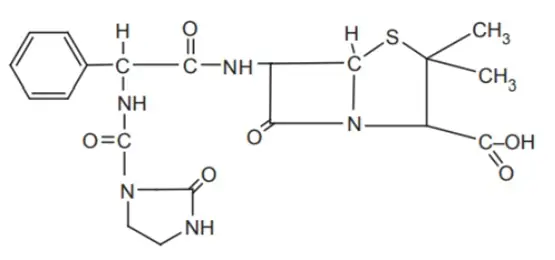
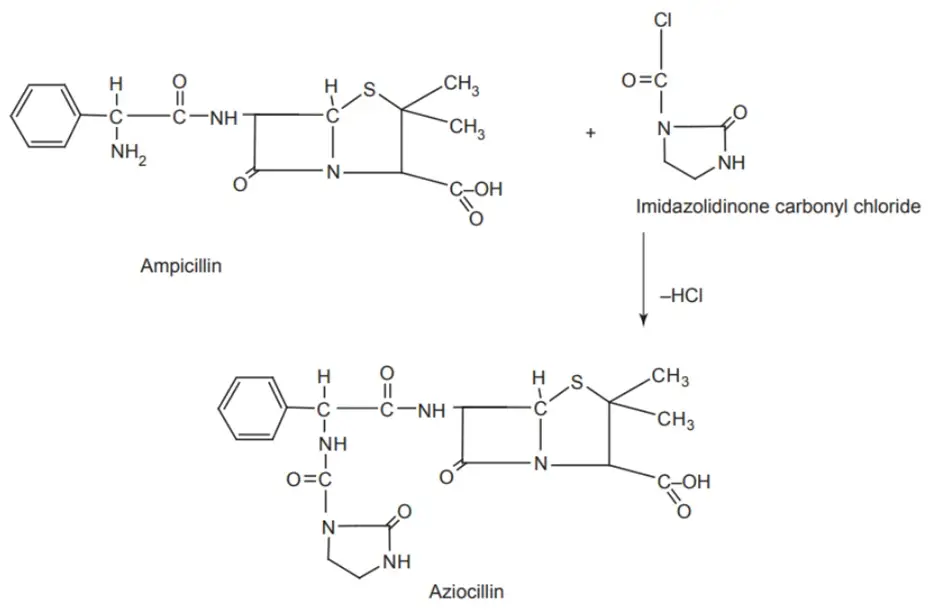
Synthesis of Aziocillin
Properties and uses
Piperacillin is a solid drug that is highly soluble in water. It is typically administered intravenously as a powder for reconstitution or as a premixed solution. Its solubility in water allows for easy preparation of intravenous formulations, making it convenient for administration in clinical settings.
Piperacillin, the newest ureidopenicillin, is about 10 times more effective against Pseudomonas and Streptococci than carbenicillin. Its broad spectrum of action makes it valuable for treating severe infections and functioning by inhibiting bacterial cell wall synthesis.
2. Piperacillin (Pipracil, Pracil)
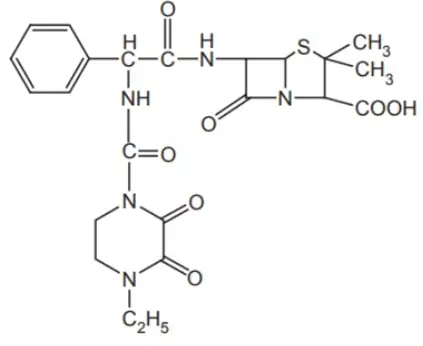
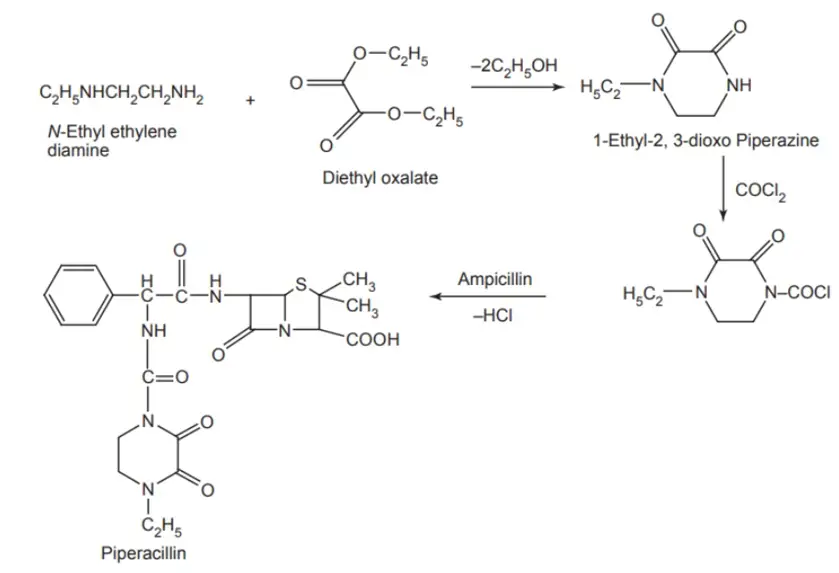
Synthesis of Piperacillin
Properties and uses
Piperacillin sodium is a white hygroscopic powder, soluble in water and methanol and practically insoluble in ethyl acetate. It is available as a powder for solubilization and injection. It is best given in combination with an aminoglycoside antibiotic.
Miscellaneous penicillins
Miscellaneous penicillins refer to penicillin derivatives that do not fall into the traditional or ureido penicillin categories. These include drugs like oxacillin, cloxacillin, and dicloxacillin, which possess a narrow spectrum of activity primarily against Gram-positive bacteria, particularly staphylococci. Unlike traditional penicillins, they resist penicillinase enzymes produced by some bacteria, making them effective against penicillin-resistant strains. Miscellaneous penicillins are commonly used to treat infections caused by Staphylococcus aureus, including skin and soft tissue infections, bone and joint infections, and certain respiratory tract infections.
1. Mecillinam (Amdinocillin)
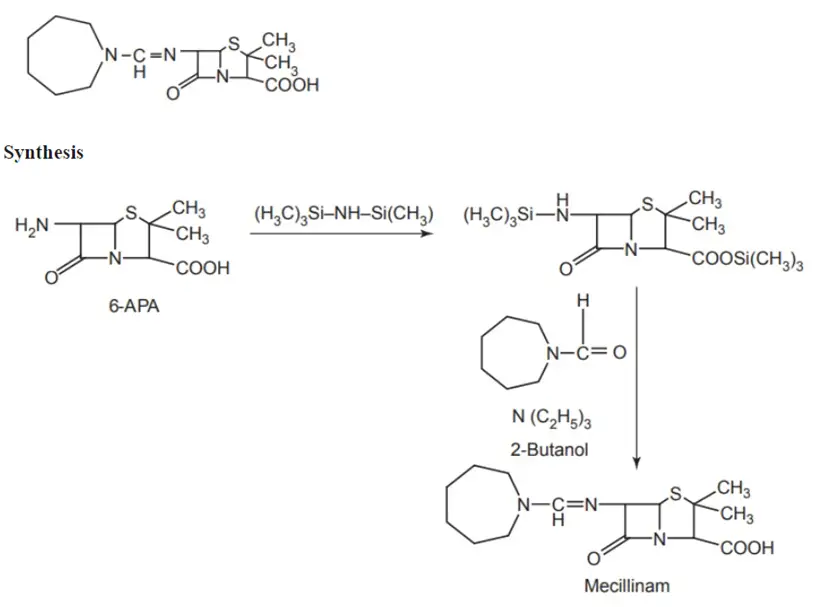
Properties and uses:
Mecillinam exhibits particular activity against enterobacteria, including some strains resistant to ampicillin, making it useful for treating urinary tract infections. Structurally distinct from other penicillins, mecillinam is not an acyl derivative but rather an alkylidene amino-(amidino) derivative of 6-APA. This difference grants it significant gram-negative antibacterial activity, while its gram-positive antibacterial activity remains comparatively lower.



