Introduction
Volhard’s method is a back titration technique used for the determination of chloride (Cl⁻), bromide (Br⁻), and iodide (I⁻) ions. It employs silver nitrate (AgNO₃) as a titrant to precipitate the halide ions, and the unreacted silver ions are then titrated with potassium thiocyanate (KSCN) using ferric ammonium sulfate as an indicator. This method is particularly useful when direct titration (such as Mohr’s method) is not feasible due to low solubility, interference, or pH sensitivity.
Principle of Volhard’s Method
Volhard’s method is based on the formation of insoluble silver halides (AgCl, AgBr, AgI) by reacting the sample solution with excess silver nitrate (AgNO₃). Back-titrate the unreacted silver ions with potassium thiocyanate (KSCN), using ferric ammonium sulfate as an indicator. Detect the endpoint by the formation of a red-colored ferric thiocyanate complex (Fe(SCN)₃).
Chemical Reactions in Volhard’s Method
Step 1: Precipitation of Chloride/Bromide/Iodide
- Treat the sample containing Cl⁻, Br⁻, or I⁻ with an excess known volume of AgNO₃.

Since AgCl, AgBr, and AgI are insoluble, they completely precipitate out of the solution.
Step 2: Back Titration with KSCN (Potassium Thiocyanate)
- The excess unreacted AgNO₃ is titrated with KSCN.

Step 3: Endpoint Detection Using Ferric Indicator
- Once the reaction consumes all Ag⁺, the next drop of KSCN reacts with ferric ammonium sulfate (Fe³⁺) to form a red-colored ferric thiocyanate complex, indicating the endpoint.
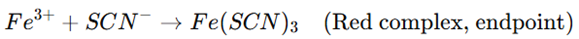
Procedure of Volhard’s Method
1. Preparation of Solutions
- Standard Silver Nitrate Solution (AgNO₃, 0.1 N): Dissolve accurately weighed AgNO₃ in distilled water and store in an amber bottle.
- Standard Potassium Thiocyanate Solution (KSCN, 0.1 N): Prepare and standardize against silver nitrate.
- Ferric Ammonium Sulfate (Indicator, 5% solution): Used to detect the endpoint.
2. Titration Procedure
- Pipette a known volume of the sample solution into a conical flask.
- Add an excess of standard AgNO₃ solution to ensure complete precipitation of halides.
- Filter the precipitate (AgCl, AgBr, or AgI) to remove it.
- Titrate the unreacted AgNO₃ in the filtrate with standard KSCN solution, using ferric ammonium sulfate as an indicator.
- Endpoint Detection: The first appearance of a persistent red color due to the ferric thiocyanate complex indicates the endpoint.
Calculation
Calculate the concentration of chloride, bromide, or iodide in the sample using:

Calculate the mass of chloride, bromide, or iodide as:

Factors Affecting Volhard’s Method
1. pH of the Solution
- The solution must be acidic (pH ≈ 2-3) to prevent hydrolysis of thiocyanate ions.
- Nitric acid (HNO₃) is used to maintain acidity.
- Higher pH (> 3) may lead to precipitation of ferric hydroxide (Fe(OH)3), interfering with endpoint detection.
2. Filtration of Precipitate
- Filter out AgCl, AgBr, or AgI before titration with KSCN.
- If left in solution, the halide precipitate may redissolve, leading to errors.
3. Indicator Interference
- Excess ferric ammonium sulfate can cause premature red coloration.
- Add the indicator only after most of the AgNO₃ has reacted.
Advantages of Volhard’s Method
- Applicable for Iodide (I⁻) and Bromide (Br⁻), Unlike Mohr’s Method
- It can be performed in acidic solutions (pH 2-3) to prevent interference.
- Highly Accurate for Low-Solubility Halides
- You can use it for sulfate and other anions that precipitate with AgNO₃.
Limitations of Volhard’s Method
- Requires Filtration of Silver Halide Precipitates (Time-Consuming)
- Sensitive to Excess Indicator, Which May Cause Early Red Color Formation
- Requires Acidic Medium, Which May Interfere with Other Ions
- Highly basic solutions cannot be used due to ferric hydroxide formation.
Applications of Volhard’s Method
- Determination of Chloride, Bromide, and Iodide in Pharmaceuticals and Water Samples
- Analysis of Salinity in Environmental Water Testing
- Determination of Halide Content in Food and Industrial Products
- Quantification of Sulfate (SO₄²⁻) Using Barium Sulfate Precipitation Followed by Titration
Comparison: Mohr’s vs. Volhard’s Method

Conclusion
Volhard’s method is a highly accurate and reliable back-titration technique for the determination of chloride, bromide, and iodide in acidic solutions. Unlike Mohr’s method, it is suitable for low-solubility halides and acidic media. However, it requires filtration of silver halide precipitates, and careful endpoint detection using ferric ammonium sulfate as an indicator.