Complexometric titration, also known as chelatometry, is a type of volumetric analysis that uses the formation of a colored complex to determine the endpoint of a titration. This method is widely used in laboratories to measure the unknown concentration of a specific analyte in a sample. It is a quantitative chemical analysis technique.
Often referred to as volumetric analysis, complexometric titration relies heavily on precise volume measurements. A reagent, called the titrant, serves as a standard solution and reacts with the analyte. The titrant volume is the amount of titrant required to complete the reaction.
This method is particularly effective for detecting and quantifying mixtures of different metal ions in a solution. With each drop of titrant added, the reaction reaches equilibrium quickly, minimizing interference and ensuring accurate results. The equivalence point is identified with high precision, making complexometric titration a reliable analytical tool.
A well-established example is the use of EDTA (ethylenediaminetetraacetic acid) as a titrant, which forms stable complexes with metal ions. This approach is widely utilized due to its accuracy and specificity.
Characteristics of Complexometric Titrations:
- The reaction is highly selective and specific.
- Requires an indicator that can exhibit a clear color change upon complex formation or dissociation.
- Usually conducted in buffered solutions to maintain a specific pH for effective complex formation.
Principle of Complexation
The principle of complexation is based on the formation of a complex, which is a stable coordination entity resulting from the interaction between a central metal ion and one or more ligands. Ligands, which can be molecules or ions, donate electron pairs to the metal ion to form coordinated covalent bonds.
Key Points:
- Coordination Bond Formation: The central metal ion acts as a Lewis acid (electron pair acceptor), while the ligands act as Lewis bases (electron pair donors).
- Stability of the Complex: Stability depends on factors such as the charge, size, and electronic configuration of the metal ion, as well as the structure and denticity of the ligand.
- Stoichiometric Ratio: Complex formation follows a specific stoichiometric ratio, such as 1:1 or 1:2, between the metal ion and the ligand, depending on their chemical properties.
- Equilibrium: The reaction reaches equilibrium when the complex is fully formed. This equilibrium is governed by the complex’s formation constant (Kf), which reflects its stability.
- Color Change: In complexometric titrations, the endpoint is detected by a color change of the metal-ligand complex, often aided by an indicator.
Example Reaction:
For a typical reaction involving EDTA and a metal ion (M²⁺):
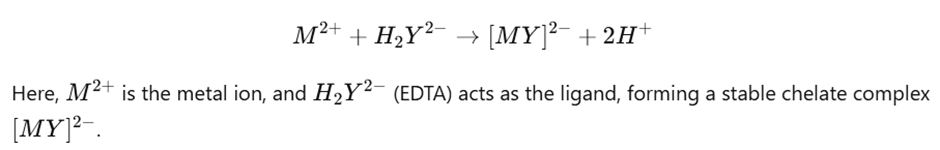
This principle underlies the use of complexation in quantitative analysis, particularly for identifying and estimating metal ions in solution.