Definition
Normality (N) is defined as the number of gram-equivalent weights of solute per liter of solution. It is a concentration unit used in titrations and chemical reactions where equivalent weight matters, such as acid-base, redox, and precipitation reactions.
Formula of Normality:

Unit:
The unit of normality is equivalents per liter (eq/L), often written as N (e.g., 0.5 N H₂SO₄).
Calculation Example:
Example:
Calculate the normality of a 1 M H₂SO₄ solution in an acid-base reaction.
Solution:

Answer: The normality of 1 M H₂SO₄ is 2 N.
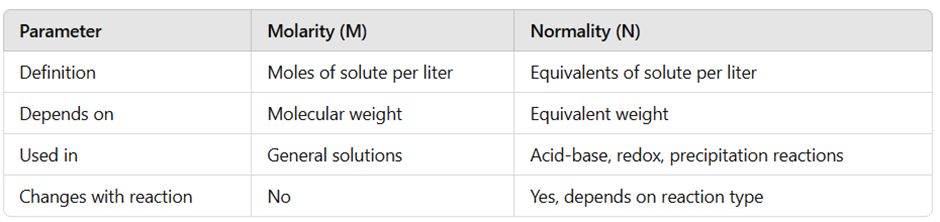
Key Differences Between Normality and Molarity
Factors Affecting Normality (N)
Normality (N) is the solution concentration expressed as equivalents of solute per liter of solution. It depends on several factors, including:
1. Amount of Solute (Equivalent Weight & Mass)
- Normality increases if the equivalent mass of solute increases.
- Normality decreases if less solute is dissolved in the same volume.
2. Volume of Solution (V)
- Increasing the volume decreases normality.
- Decreasing the volume increases normality.
3. Temperature
- It affects the volume of the solution, thus altering normality.
- Higher temperatures expand the solution, lowering normality.
4. Valency Factor (n-factor or Equivalent Factor)
Different solutes have different valency factors based on their reaction type:
- Acids: Normality depends on the number of replaceable H⁺ ions (e.g., H₂SO₄ has n = 2).
- Bases: Normality depends on the number of OH⁻ ions (e.g., NaOH has n = 1, Ca(OH)₂ has n = 2).
- Salts: Normality is affected by ion exchange capacity in reactions.
- Redox Reactions: Normality depends on electron exchange per mole.
5. Dissociation & Ionization
- Strong acids/bases fully ionize, affecting the number of equivalents in the solution.
- Weak acids/bases partially ionize, reducing the effective concentration.
Applications of Normality in Pharmaceuticals
- Titration calculations: Normality is useful in acid-base titrations where the number of H⁺ or OH⁻ ions determines the reaction.
- Redox reactions: Normality helps in balancing oxidation-reduction reactions.
- Precipitation reactions: Used in solutions where ions react in equivalent proportions.
- Pharmaceutical quality control: helps in determining the strength of acids, bases, and salts in formulations.
Conclusion
Normality is an essential concentration unit for quantitative chemical analysis, especially in titrations and redox reactions. It provides a more precise measurement than molarity when dealing with acids, bases, and oxidizing or reducing agents. Understanding normality is crucial in pharmaceutical, chemical, and clinical laboratories for accurate solution preparation and standardization.