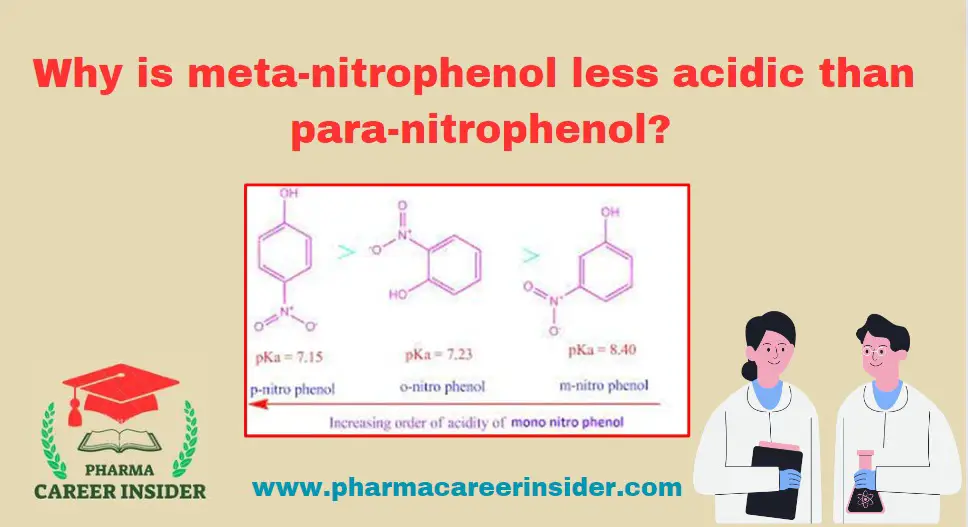
The position of the substituent on the aromatic ring influences the acidity of substituted phenols. In the case of meta-nitrophenol (m-nitrophenol) and para-nitrophenol (p-nitrophenol), the nitro group (NO2) is the substituent.
The key factor influencing acidity in substituted phenols is stabilizing the phenoxide ion formed during the deprotonation process. Let’s compare the acidity of m-nitrophenol and p-nitrophenol:
1. Para-Nitrophenol (p-Nitrophenol):
In p-nitrophenol, the (NO2) group is in the para position. The negative charge on the phenoxide ion formed during deprotonation can resonate between the oxygen and nitrogen atoms of the (NO2) group. This resonance stabilization enhances the acidity of p-nitrophenol.
2. Meta-Nitrophenol (m-Nitrophenol):
In m-nitrophenol, the (NO2) group is in the meta position.The negative charge on the phenoxide ion has limited resonance possibilities in the meta position compared to the para position.The resonance stabilization in m-nitrophenol is less effective, leading to lower acidity than p-nitrophenol.
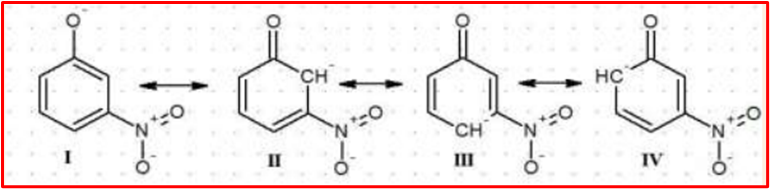
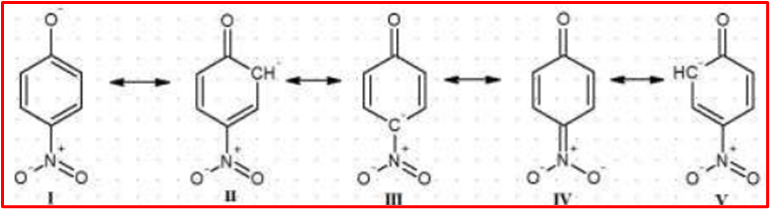
In summary, the para position allows for more effective resonance stabilization of the phenoxide ion, making para-nitrophenol more acidic than meta-nitrophenol. The resonance effects play a crucial role in determining the relative acidity of substituted phenols.