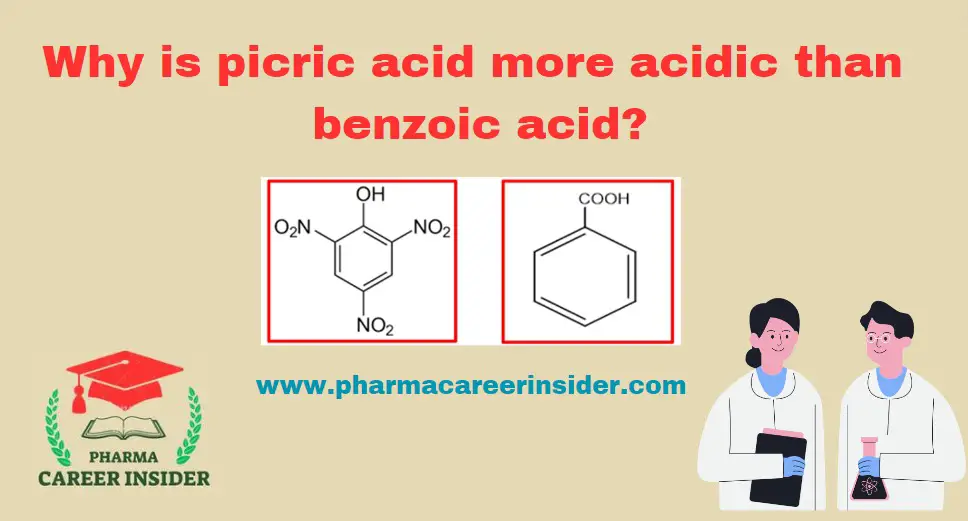
The acidity of carboxylic acids is generally attributed to the stability of the conjugate base (carboxylate ion) formed after the loss of a proton. In the case of picric acid (2,4,6-trinitrophenol) and benzoic acid, the substituents on the aromatic ring play a crucial role in determining acidity.
Here’s a comparison of picric acid and benzoic acid:
1. Picric Acid (2,4,6-Trinitrophenol):
Picric acid contains three nitro (NO2) groups on the aromatic ring as substituents. The presence of multiple electron-withdrawing nitro groups increases the acidity of picric acid. The negative charge on the picrate ion formed during deprotonation is delocalized over the three nitro groups through resonance, leading to enhanced stability.
2. Benzoic Acid:
Benzoic acid contains a carboxyl (COOH) group on the aromatic ring as a substituent.
While the carboxyl group is electron-withdrawing, it is not as strongly electron-withdrawing as the nitro groups in picric acid. The stability of the benzoate ion formed after deprotonation is not as pronounced as in picric acid due to less effective resonance effects.
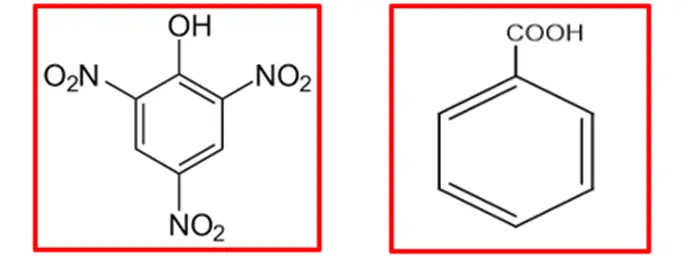
In summary, the greater acidity of picric acid compared to benzoic acid can be attributed to three strongly electron-withdrawing nitro groups, which enhance resonance stabilization to the conjugate base. The multiple nitro groups in picric acid contribute to its higher acidity.