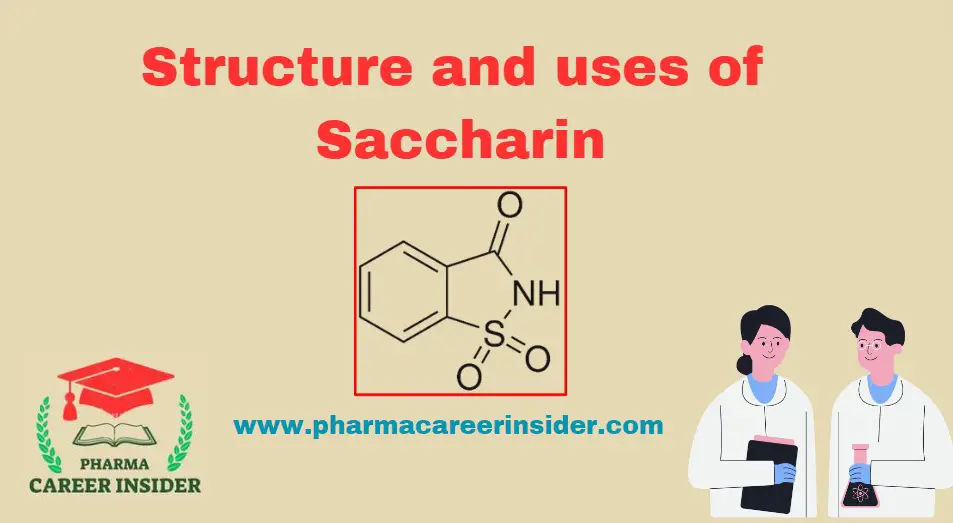
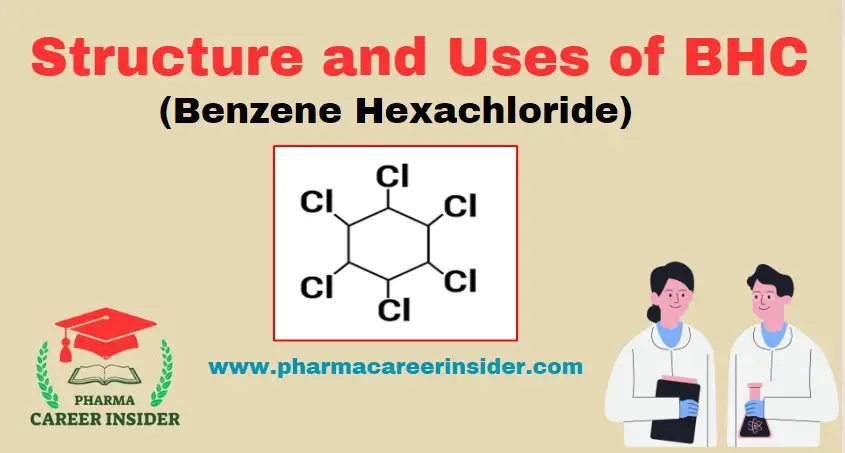
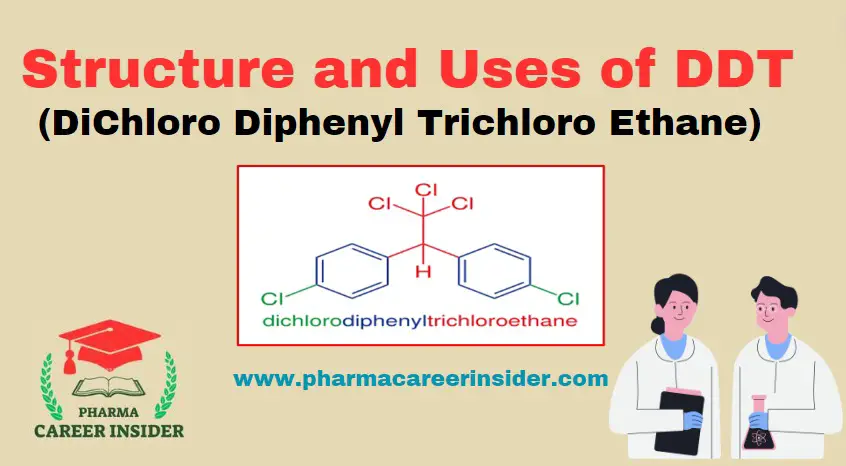
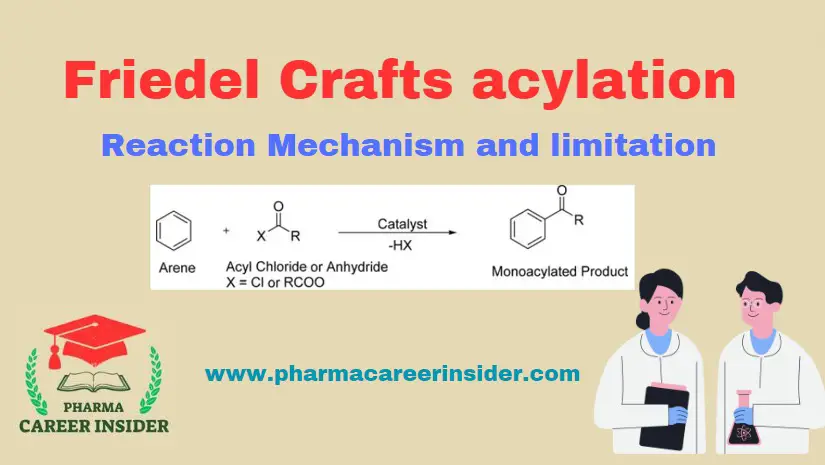
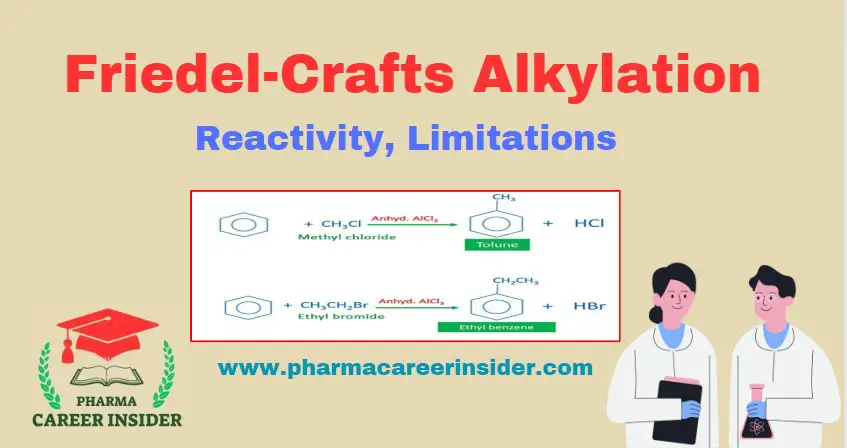
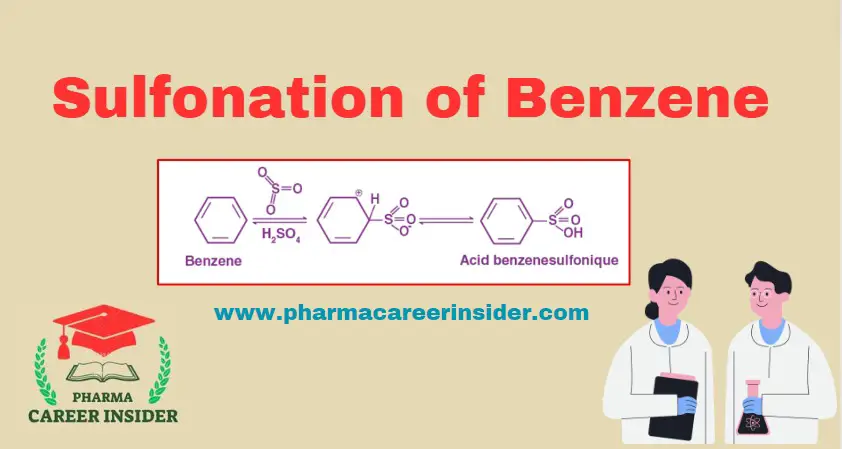
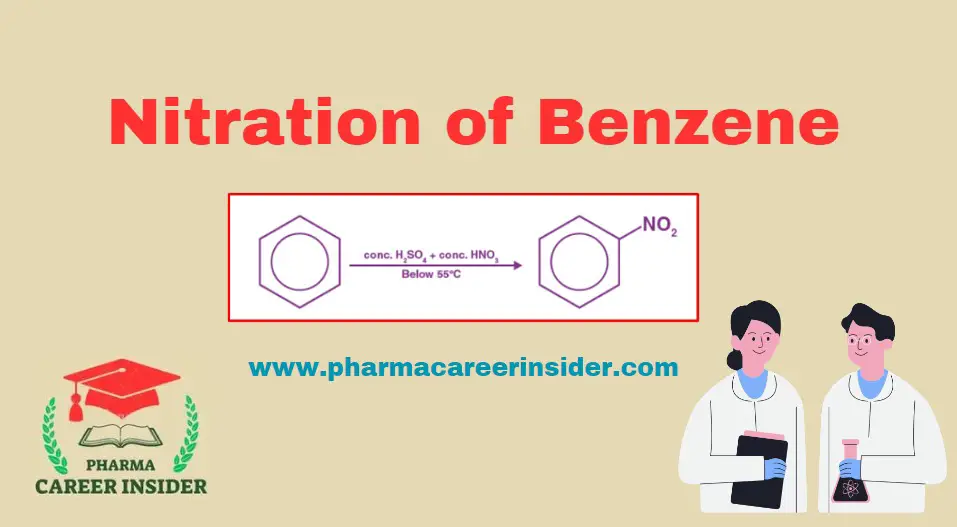
Structure and uses of Saccharin
Structure: IUPAC Name: 2H-1λ6,2-benzothiazol-1,1,3-trione Molecular Formula: C7H5NO3S Properties: Uses: 1. Food and Beverages: Saccharin is commonly used as a low-calorie artificial sweetener in various food and beverage products, providing sweetness without adding significant calories. 2. Tabletop Sweeteners: They are employed in tabletop sweeteners for individuals seeking a sugar-free or low-calorie alternative for sweetening drinks and … Read more