Introduction
Precipitation titrations are a type of volumetric analysis in which the reaction between the analyte and the titrant results in the formation of an insoluble precipitate. These titrations are commonly used to determine the concentration of ions that form sparingly soluble salts with the titrant. The most common examples include the determination of halide ions (Cl⁻, Br⁻, I⁻) using silver nitrate (AgNO₃), known as argentometric titrations.
Principle of Precipitation Titration
Precipitation titrations rely on the principle that an insoluble precipitate forms when a titrant is added to a solution containing an analyte. The reaction continues until the equivalence point is reached, at which all of the analyte reacts with the titrant. An indicator is often used to detect the endpoint. The precipitate’s solubility product constant (Ksp) plays a crucial role in determining the feasibility and accuracy of precipitation titrations. A lower Ksp value ensures better precipitation and a sharper endpoint.
Types of Precipitation Titrations
1. Argentometric Titrations
These titrations involve silver nitrate (AgNO₃) as a titrant and are used mainly for halide ion determinations.
- Mohr’s Method: It uses potassium chromate (K₂CrO₄) as an indicator to determine chloride (Cl⁻) and bromide (Br⁻) ions.
- Volhard’s Method: Uses ferric ammonium sulfate as an indicator for back-titration of chloride, bromide, and iodide ions.
- Fajans Method: Uses adsorption indicators such as fluorescein to detect the endpoint.
2. Mercurimetric Titrations
Mercuric nitrate (Hg(NO₃)₂) is used as a titrant, often for chloride (Cl⁻) determination.
Methods of Detecting Endpoints
1. Mohr’s Method (Direct Titration)
- Used for Cl⁻ and Br⁻ determination.
- Reaction:
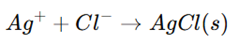
- Potassium chromate (K₂CrO₄) is used as an indicator.
- When all Cl⁻ is precipitated, excess Ag⁺ reacts with chromate, forming a red precipitate of silver chromate (Ag₂CrO₄), indicating the endpoint.
2. Volhard’s Method (Back Titration)
- Used for Cl⁻, Br⁻, and I⁻ determination.
- Reaction:
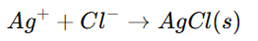
- Add excess AgNO₃ and then back-titrate with potassium thiocyanate (KSCN).
- Ferric ammonium sulfate acts as an indicator. When all Ag⁺ is consumed, Fe³⁺ reacts with SCN⁻ to form a red complex, indicating the endpoint.
3. Fajans Method (Adsorption Indicator Method)
- Used for Cl⁻ determination.
- Reaction:

- A dye (e.g., dichlorofluorescein) is used as an adsorption indicator.
- Before the endpoint, the AgCl precipitate is negatively charged. At the endpoint, excess Ag⁺ ions cause the precipitate to acquire a positive charge, leading to the adsorption of the dye and a color change.
Factors Affecting Precipitation Titrations
- Solubility Product (Ksp): Lower Ksp ensures sharp endpoints.
- Titrant Concentration: Higher concentrations lead to better precipitation.
- pH of Solution: Affects solubility and ionization of the precipitate.
- Temperature: Higher temperatures may increase solubility, affecting precipitation.
- Presence of Complexing Agents: This may interfere with precipitation by forming soluble complexes.
Applications of Precipitation Titrations
- Determination of chloride, bromide, and iodide in pharmaceuticals and water samples.
- Analysis of sulfate using barium chloride (BaCl₂).
- Determination of cyanide (CN⁻) and thiocyanate (SCN⁻) in industrial samples.
- Estimation of silver content in alloys.
Conclusion
Precipitation titrations are a vital analytical technique for quantifying ions that form sparingly soluble precipitates. Choose the method (Mohr, Volhard, or Fajans) based on the ion being analyzed and the required accuracy. Understanding factors like solubility products, indicators, and endpoint detection is crucial for achieving precise results.