Introduction:
Electrophilic aromatic substitution reactions are a category of organic reactions in which an electrophile displaces an atom bonded to an aromatic ring. Typically, these reactions entail the substitution of a hydrogen atom within a benzene ring with an electrophile.
In electrophilic aromatic substitution, the aromaticity of the ring remains intact. For instance, the formation of bromobenzene from the reaction between benzene and bromine does not compromise the stability of the aromatic system. The reaction can be depicted as follows.
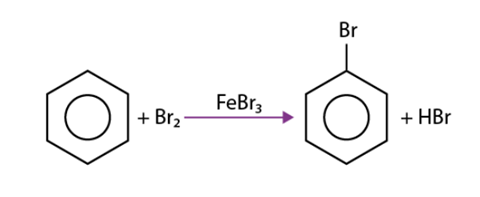
Aryl halides, or haloarenes, can be synthesized through electrophilic aromatic halogenation reactions, wherein aromatic rings react with iodine, chlorine, or bromine. These reactions typically employ aluminium trihalides as catalysts to facilitate the halogenation process.
Electrophilic Aromatic Substitution Reaction
There exist many types of electrophilic aromatic substitution reactions, the most important of which include:
- Aromatic nitration reactions
- Electrophilic aromatic halogenation reactions
- Aromatic sulfonation reactions
- Friedel-Crafts alkylation reaction
- Friedel-Crafts acylation reaction
The aromatic system used as a reactant in these reactions is usually benzene.