Carboxylic acids can be prepared through various methods, including:
1. Oxidation of Primary Alcohols or Aldehydes: Primary alcohols can be oxidized to carboxylic acids using oxidizing agents such as potassium permanganate (KMnO4) or chromium trioxide (CrO3) in acidic or alkaline conditions. Aldehydes can also be oxidized to carboxylic acids under similar conditions.

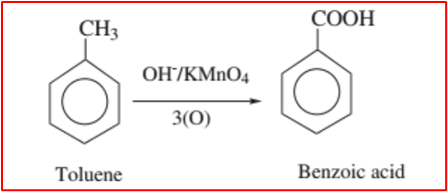
2. Oxidation of Alkylbenzenes: Alkylbenzenes, such as toluene, can be oxidized to benzoic acid using strong oxidizing agents like chromyl chloride (CrO2Cl2) or potassium permanganate (KMnO4) in the presence of sulfuric acid.
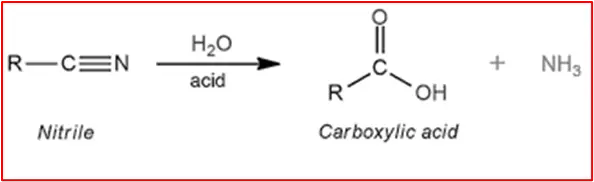
3. Hydrolysis of Nitriles: Nitriles can be hydrolyzed under acidic or basic conditions to yield carboxylic acids. The acidic hydrolysis of nitriles involves the use of dilute sulfuric acid, while basic hydrolysis uses a strong base like sodium hydroxide.
4. Kolbe’s Electrolysis of Salts of Carboxylic Acids: Salts of carboxylic acids, known as carboxylates, can undergo Kolbe’s electrolysis to yield carboxylic acids. This method involves the electrolysis of the sodium or potassium salt of the carboxylic acid in aqueous solution.
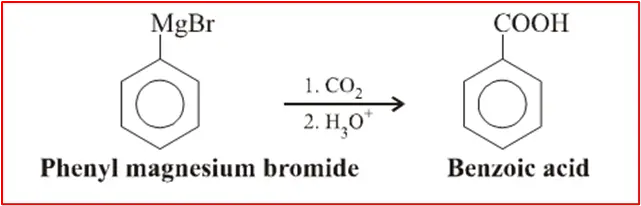
5. Grignard Reaction with Carbon Dioxide: Organomagnesium compounds, known as Grignard reagents, can react with carbon dioxide (CO2) to form carboxylic acids. The reaction involves the addition of a Grignard reagent to dry ice (solid CO2) or bubbling CO2 gas through an ether solution of the Grignard reagent.
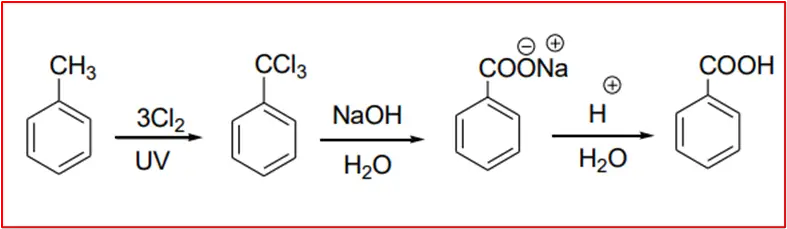
6. By basic hydrolysis of benzo trichloride: Benzo trichloride is obtained by chlorination of toluene.
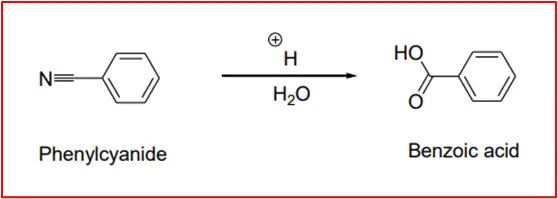
7. Hydrolysis of Phenyl Cyanide: Obtaining Benzoic Acid through the Acid Hydrolysis of Phenyl Cyanide.