Introduction:
Phenols are organic compounds containing a benzene ring bonded to a hydroxyl group. They are also known as carbolic acids. Phenols react with active metals like sodium and potassium to form phenoxide. This reaction of phenol with metals indicates its acidic nature. Compared to alcohols, phenols are more potent acids as the pKa value for phenol is 10, while for alcohols, it’s close to. Because of its acidic nature, phenols can turn blue litmus red and react with aqueous alkali to form phenate. Both reactions are not shown by alcohol. Compared to carboxylic acids, phenols are weaker acids.
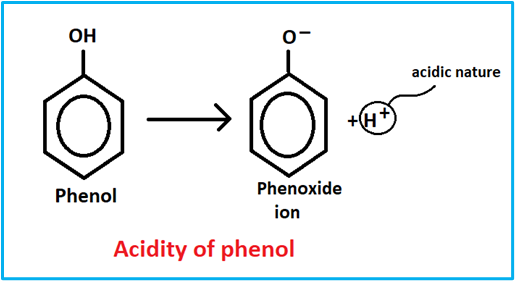
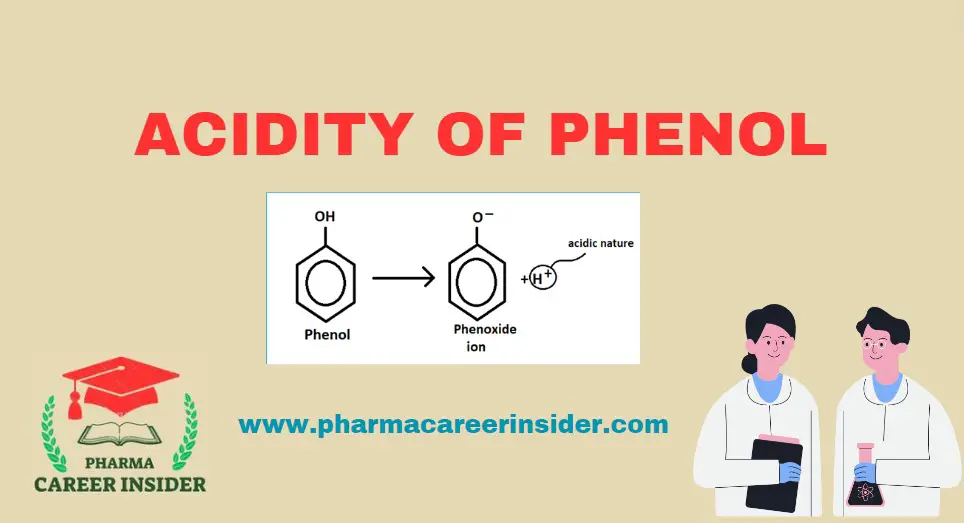
Hence, the hydroxy group bonded to the benzene ring is much more acidic than a hydroxy group to alcohol. This is because of the resonance in phenol. Because of the resonance in phenol, the oxygen atom acquires a positive charge, which weakens the oxygen-hydrogen bond and facilitates the release of a proton.

The deprotonation of phenol forms phenoxide ion or phenate, which also exists as a resonance.
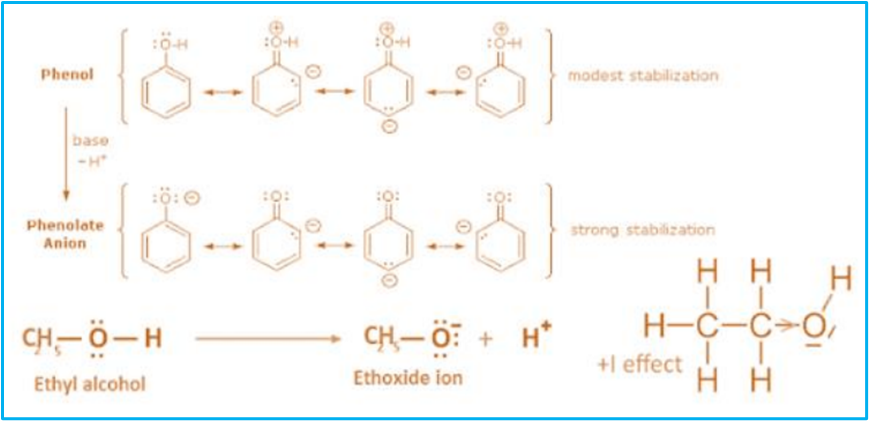
Hence, both phenol and phenoxide ions are stabilized by resonance. Compared to phenol, phenoxide ion is more stable as the negative charge delocalizes over the benzene ring. However, the resonating structure of phenol involves separating negative and positive charges. Therefore, phenol has a greater tendency to form phenate by releasing the proton. The deprotonation of alcohol forms alkoxide ions, which are not stable due to the negative inductive effect of the alkyl group, and alcohols become less acidic than phenol.
In ethoxide, resonance is not possible, and the + I effect of the hydrocarbon chain increases the electron density between O and H and decreases the chances of H + leaving the alcohol. The longer and branched the chain, the greater the effect, and the less acidic the alcohol will be acid.