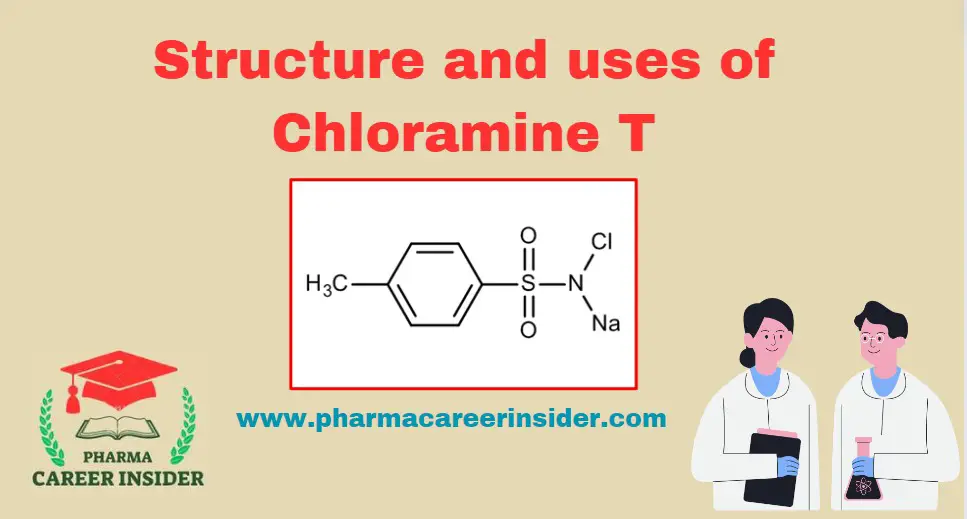
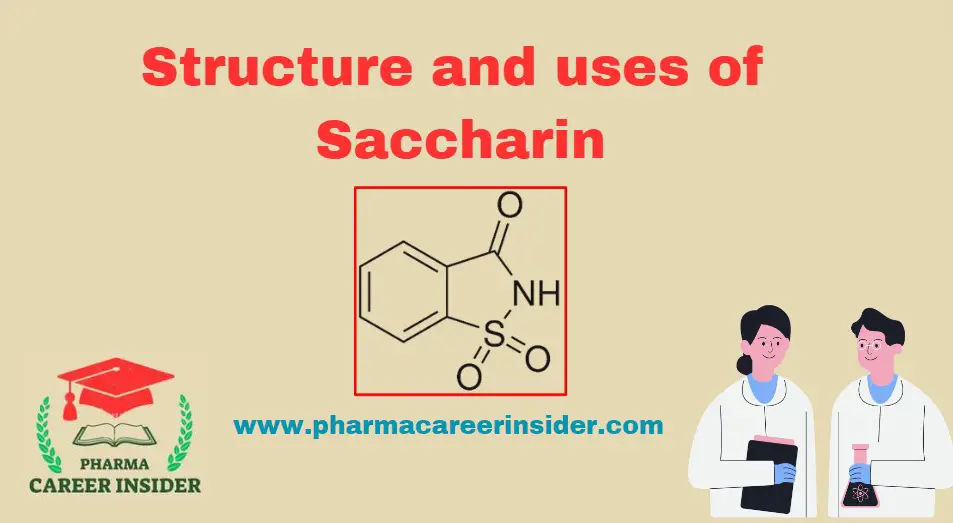
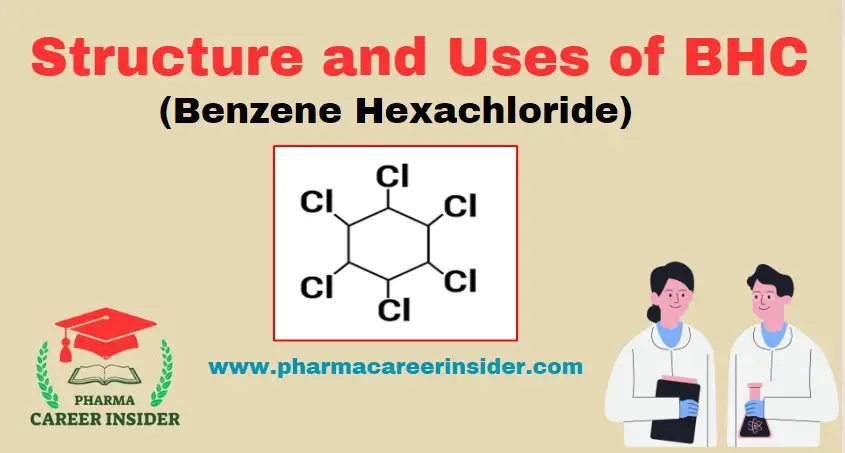
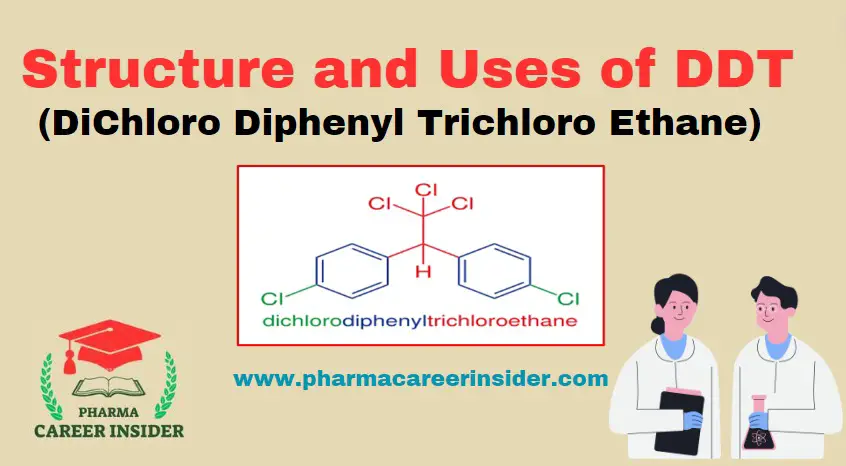
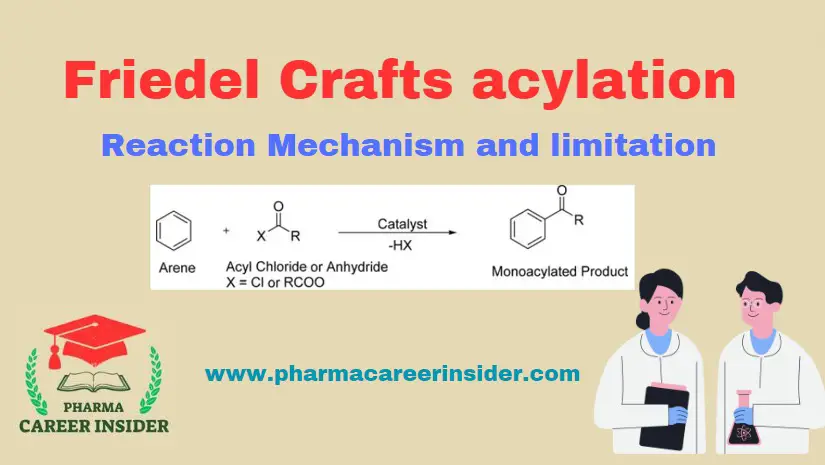
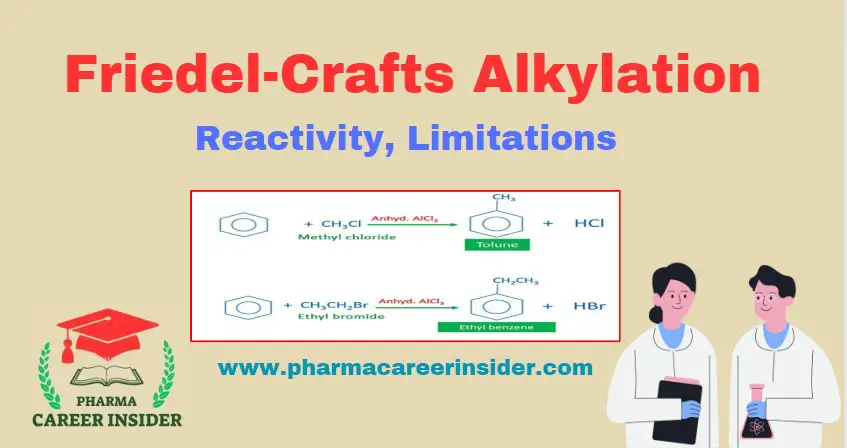
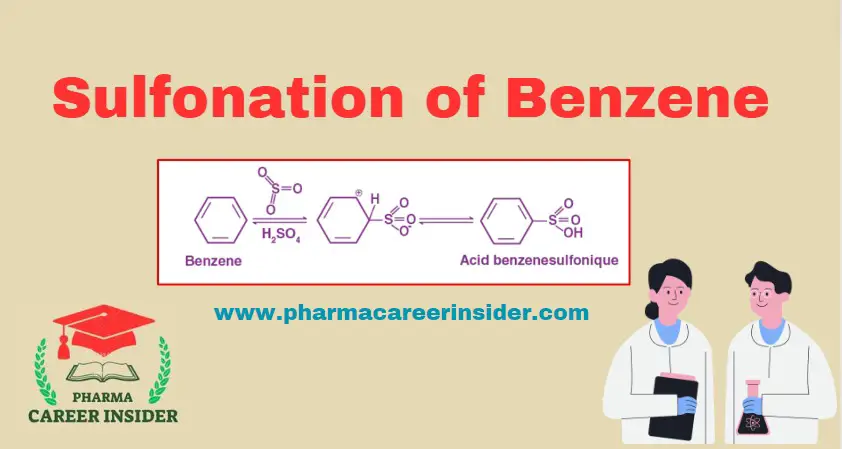
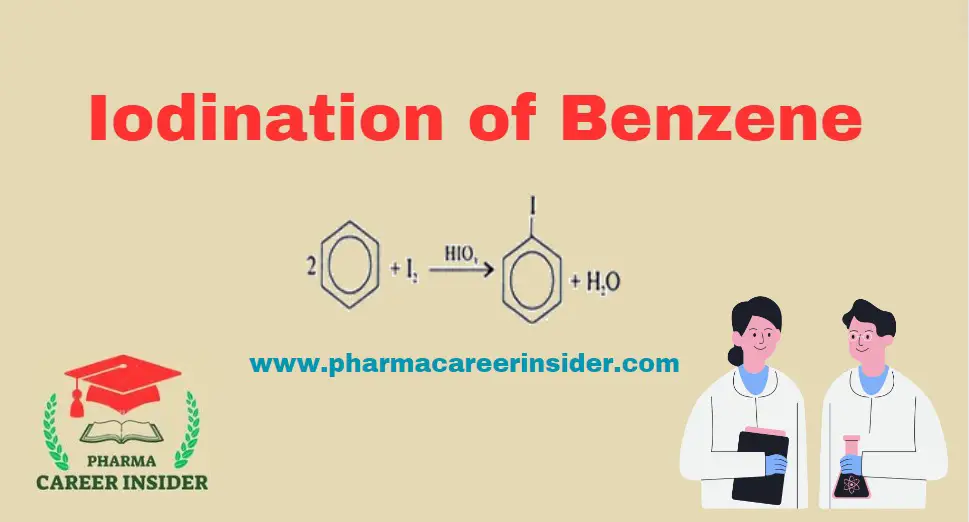
Structure and uses of Chloramine T
Structure: Properties: Tosylchloramide, also known as chloramine-T, is a chlorinated and deprotonated sulfonamide used as a mild disinfectant. It is not stable in the water-dissolved form. Uses: 1. Disinfection in Water Treatment: Chloramine T is employed as a water disinfectant in water treatment processes, helping to control and eliminate harmful microorganisms. 2. Veterinary Antiseptic: Used … Read more