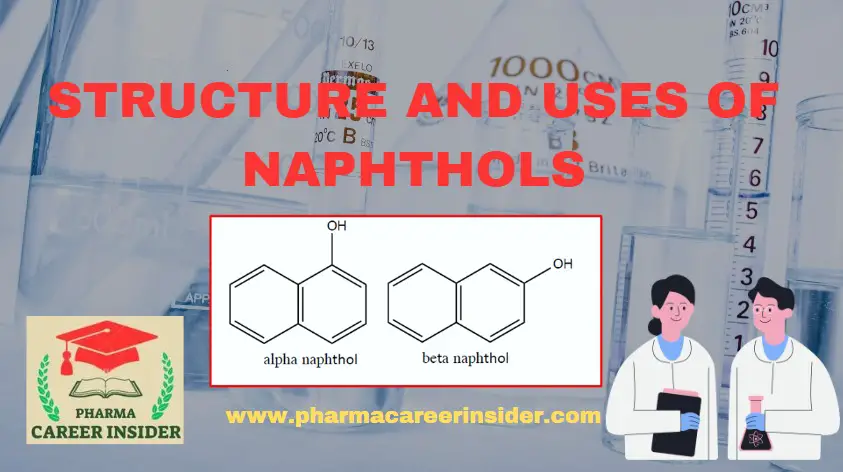
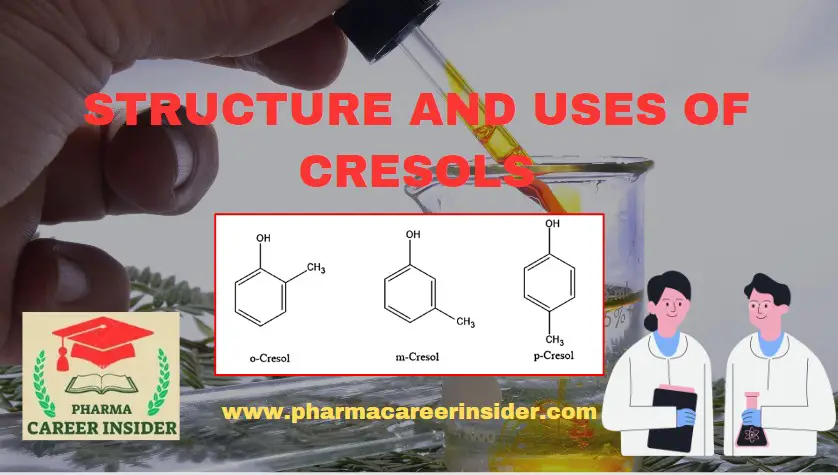
Physical properties of Aromatic amines
Aromatic amines, derivatives of benzene with amino (-NH2) functional groups, exhibit distinct physical properties. Here are some key physical properties of aromatic amines: 1. State at Room Temperature: – Aromatic amines are generally colourless liquids or solids at room temperature. – The state (solid or liquid) depends on factors such as molecular size, … Read more