(A). Reactions of -OH group:
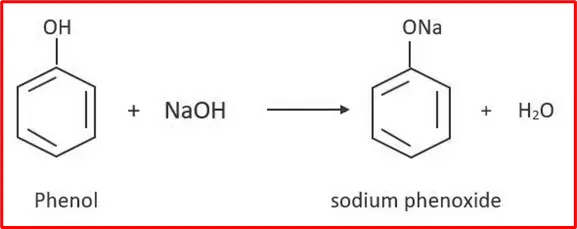
1. Formation of Salts: Phenol is acidic. It reacts with sodium hydroxide or sodium metal to form salts.
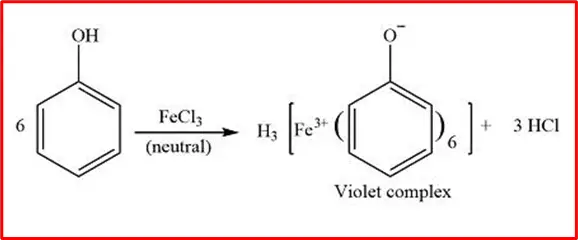
2. Reaction with FeCl3: Phenol, when exposed to a neutral FeCl3 solution, produces a soluble violet-coloured complex in water.
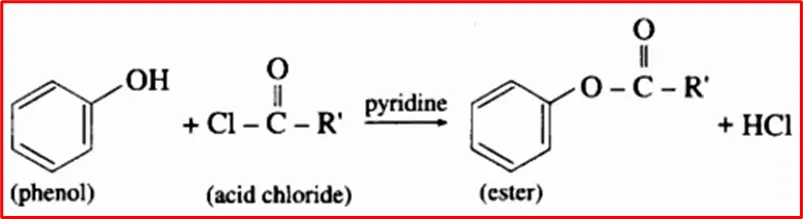
3. Formation of Esters: Phenol reacts with acid chlorides in an aqueous alkali (pyridine) solution, yielding phenyl esters.
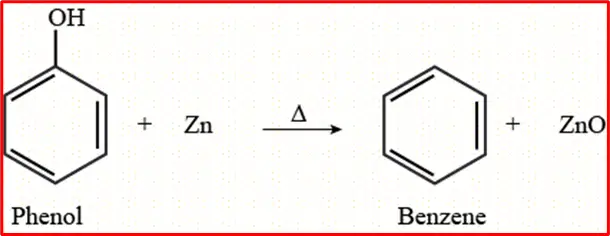
4. Reaction with Zinc Dust: A low benzene yield is obtained when phenol is distilled with zinc dust.
(B). Reactions of Benzene Ring:
The hydroxy group in a phenol molecule exerts a potent activating effect on the benzene ring by serving as a readily available source of electron density. This directing influence is robust enough that substitutions on phenols can often be achieved without a catalyst.
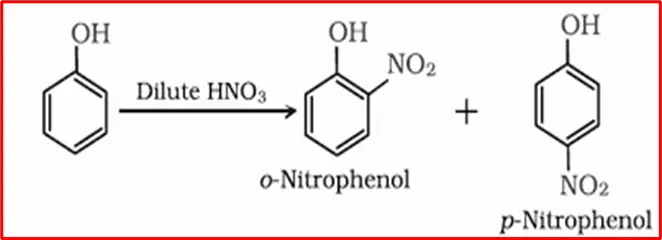
1. Nitration of Phenols: Phenols, when exposed to dilute nitric acid at low temperature (298 K), undergo nitration, producing a blend of ortho and para nitrophenols. Based on their volatility, the resultant mixture is subsequently separated into ortho and para nitrophenols through steam distillation.
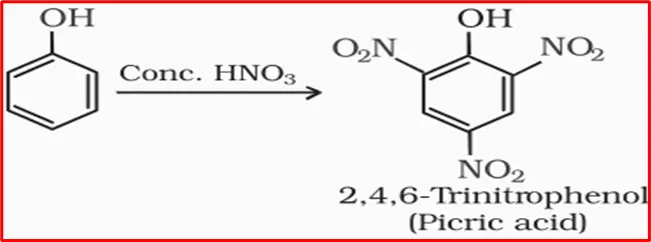
When phenol is treated with concentrated nitric acid, the nitration forms 2, 4, 6-trinitrophenol (commonly called picric acid).
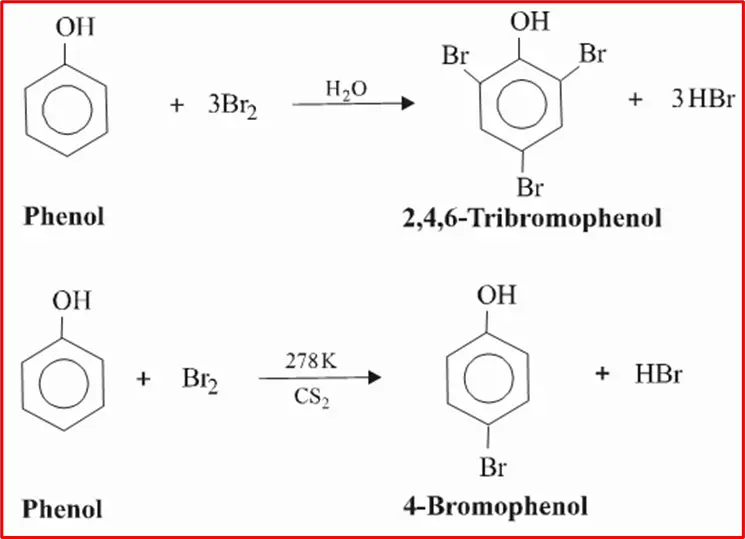
2. Halogenation of Phenols: The hydroxyl group’s highly activating effect in phenols facilitates halogenation, even without Lewis’s acids. When phenol is treated with bromine water, a white precipitate of 2, 4, 6 -tribromophenol is formed.
When phenols are exposed to bromine in a low-polar solvent such as CHCl3 at low temperatures, mono bromophenols are produced.
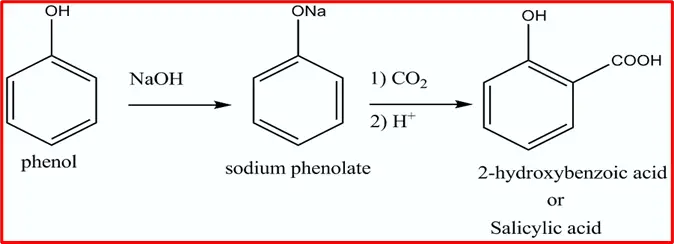
3. Kolbe’s Reaction: When phenol reacts with sodium hydroxide, it forms the highly reactive phenoxide ion. This ion, known for its reactivity in electrophilic substitution reactions, undergoes a reaction with a weak electrophile, such as carbon dioxide. This electrophilic substitution results in the formation of ortho-hydroxybenzoic acid, a process commonly referred to as Kolbe’s reaction.
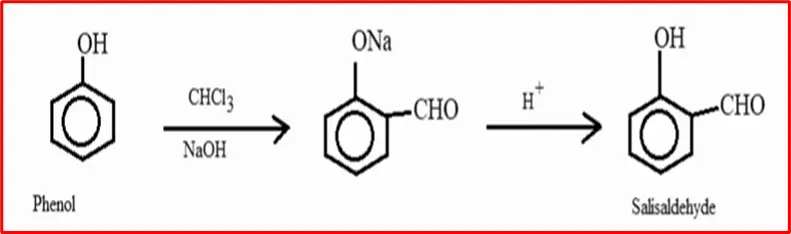
4. Reimer-Tieman Reaction: When phenol undergoes treatment with chloroform in the presence of sodium hydroxide, it forms an aldehyde group positioned at the ortho position of the benzene ring. This chemical transformation is commonly referred to as the Reimer-Tieman reaction.
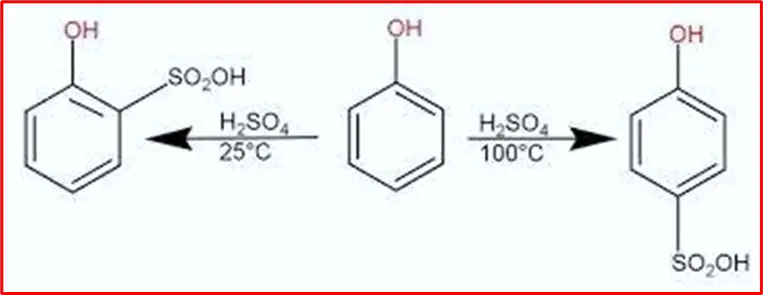
5. Sulphonation of phenols: The sulphonation of phenol occurs readily, resulting in either the ortho isomer or para isomer depending on the temperature. The ortho isomer predominates at low temperatures, while the para-isomer is formed at high temperatures (100oC).
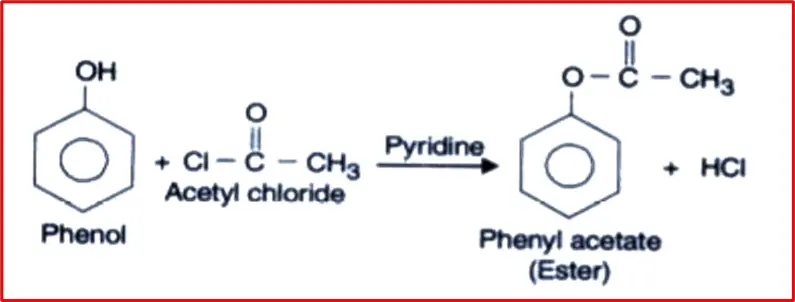
6. The acetylation reaction of phenol: The acetylation reaction of phenol involves introducing an acetyl group (CH3CO-) onto the phenol molecule’s hydroxyl (-OH) group. This reaction is commonly carried out using acetic anhydride (or acetyl chloride) in the presence of a catalyst, such as concentrated sulfuric acid/Pyridine, to form Phenylacetate (Ester).
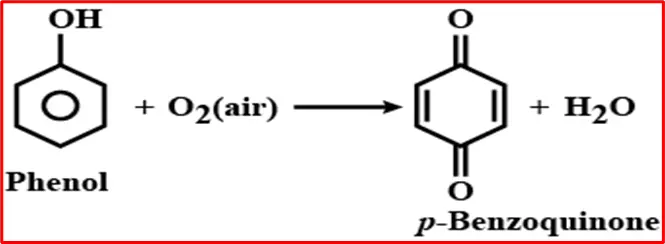
7. Oxidation of phenol: Upon exposure to air, phenol undergoes a gradual oxidation process, forming a pink-colored compound known as p-benzoquinone.
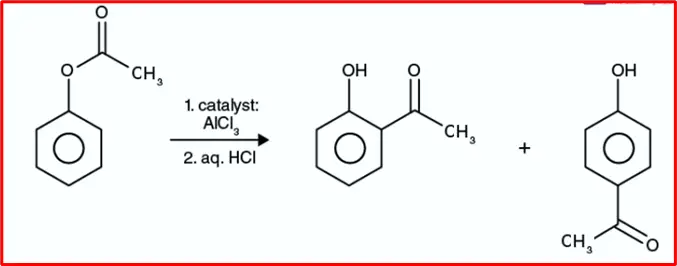
8. Fries Rearrangement Reaction: The Fries Rearrangement is an organic rearrangement reaction where an aryl ester transforms a hydroxy aryl ketone. This process typically involves using a Lewis acid catalyst and an aqueous acid.