1. Introduction
Dichrometry is a type of volumetric analysis based on the use of dichromate ions (Cr₂O₇²⁻) as the titrant in redox titrations. The dichromate ion is a strong oxidizing agent and is typically used to determine the concentration of reducing agents, particularly in acidic solutions. Dichrometry is commonly used in the determination of substances like iron (II) ions, hydrogen peroxide, and certain organic compounds that act as reducing agents.
In a dichrometric titration, potassium dichromate (K₂Cr₂O₇) or sodium dichromate (Na₂Cr₂O₇) is the source of dichromate ions. The reaction involves the reduction of dichromate ions (Cr₂O₇²⁻) to chromium (III) ions (Cr³⁺), while the reducing agent in the sample is oxidized.
2. Principle of Dichrometry
The principle of dichrometry is based on the redox reaction between dichromate ions (Cr₂O₇²⁻) and a reducing agent. The dichromate ions act as the oxidizing agent, and they are reduced to chromium (III) ions (Cr³⁺) in the process. The reducing agent donates electrons to the dichromate ions, which undergo a reduction.
- Reduction of dichromate ions (Cr₂O₇²⁻):
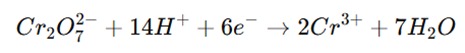
The reducing agent is oxidized in the process, and its concentration is determined by the amount of dichromate used in the titration. The endpoint of the titration is typically detected using a suitable indicator, such as diphenylamine, which gives a distinct color change when all the reducing agent has reacted.
3. Procedure of Dichrometry
The following steps outline the typical procedure for performing a dichrometry titration:
Preparation of the sample
- The sample solution containing the reducing agent to be determined is prepared. If necessary, dilute or dissolve the sample in a suitable solvent.
- The sample should be acidic to ensure that the dichromate ion remains in its effective oxidizing state (Cr₂O₇²⁻). Usually, dilute sulfuric acid (H₂SO₄) is added to acidify the solution.
Preparation of the dichromate solution
A standard solution of potassium dichromate (K₂Cr₂O₇) or sodium dichromate (Na₂Cr₂O₇) is used as the titrant. The concentration of the dichromate solution is usually determined by a standardization process, often using a primary standard like iron (II) ammonium sulfate (Fe(NH₄)₂(SO₄)₂).
Titration
- Fill a burette with the standard dichromate solution.
- Add a known volume of the sample solution to a conical flask.
- Add a few drops of an indicator, such as diphenylamine (which changes color from colorless to blue), to the sample solution.
Titration process
- Slowly titrate the sample with the dichromate solution while stirring the solution constantly.
- The titration continues until the solution changes color, indicating the endpoint. The endpoint corresponds to the complete oxidation of the reducing agent and the reduction of dichromate ions to chromium (III).
Calculation
The concentration of the reducing agent in the sample can be calculated using the volume of dichromate solution required for titration and its concentration.
The general formula for calculating the concentration of the reducing agent is:

4. Applications of Dichrometry:
Dichrometry is widely used in chemical and environmental analysis for determining the concentration of various reducing agents. Some key applications include:
- Determination of Iron (II) (Fe²⁺) Ions: One of the most common applications of dichrometry is the determination of iron (II) (Fe²⁺) ions. Iron (II) acts as a reducing agent, and its concentration can be determined by titration with a standard solution of potassium dichromate.
- Analysis of Hydrogen Peroxide (H₂O₂): Hydrogen peroxide is another reducing agent that can be determined using dichrometry. The dichromate solution oxidizes hydrogen peroxide, and the amount of dichromate consumed is directly related to the concentration of hydrogen peroxide.
- Determination of Organic Compounds: Dichrometry can be used for the determination of organic compounds that act as reducing agents, such as aldehydes, alcohols, and some organic acids. These compounds are oxidized by dichromate, and the reduction of dichromate is used to calculate their concentration.
- Environmental and Water Testing: Dichrometry is used in the analysis of water for various reducing substances, such as sulfur dioxide (SO₂) or organic materials that may act as reducing agents. It is used in monitoring environmental pollution levels.
- Determination of Copper (I): Dichrometry titration is used for the determination of copper (I) ions in solutions, where copper (I) is oxidized to copper (II) and dichromate is reduced to chromium (III).
- Analysis of Oxidizing Agents in Food and Beverages: Dichrometry is sometimes used in food and beverage analysis to quantify substances like sulfur dioxide or certain preservatives that act as reducing agents.
5. Advantages and limitations of Dichrometric Titration
Advantages:
- Accurate and precise: Dichrometry provides accurate and reliable results, particularly for determining reducing agents in the presence of interfering substances.
- Simple procedure: The titration method is straightforward and does not require complex equipment.
- Wide applicability: It applies to a wide range of reducing agents, including both inorganic and organic substances.
- Well-established technique: The method is well-established and has been used for many years in various analytical applications.
Limitations:
- Interference from other reducing agents: Other substances that act as reducing agents may interfere with the titration, affecting the results. Care must be taken to avoid such interference.
- Sensitivity to acidity: The titration requires an acidic medium to maintain the stability of the dichromate ion, which may limit its application in non-acidic samples.
- Handling of dichromate: Potassium dichromate is toxic and should be handled with care. It also requires careful disposal due to its environmental impact.
In summary, dichrometry is an effective and reliable method for determining the concentration of reducing agents, especially iron (II), hydrogen peroxide, and various organic compounds. By using potassium dichromate or sodium dichromate as the titrant, dichrometry plays a vital role in chemical, environmental, and pharmaceutical analyses.





Intresting post.
Keep updating team.
Thank you for feedback.