Introduction:
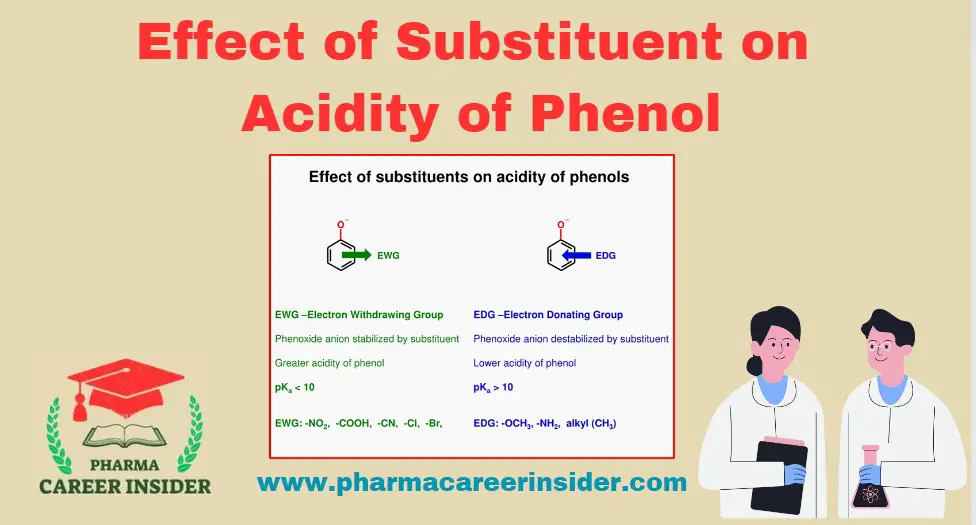
The acidity of phenol is influenced by the nature and position of substituents on the aromatic ring. Substituents can either enhance or diminish the acidity of phenol. Here’s a breakdown of the effect of substituents on the acidity of phenol:
1. Electron-Withdrawing Substituents (EWS):
Effect: Substituents such as nitro (NO2), carbonyl (COR), and sulfonyl (SO3H) groups withdraw electron density from the aromatic ring.
Impact: The presence of electron-withdrawing substituents increases the acidity of phenol.
Reason: Electron withdrawal stabilizes the phenoxide ion formed during ionization.
2. Electron-Donating Substituents (EDS):
Effect: Substituents like alkyl (CH3), (C2H5), amino (NH2), and hydroxyl (OH) groups donate electron density to the aromatic ring.
Impact: The presence of electron-donating substituents decreases the acidity of phenol.
Reason: Electron donation hinders the stabilization of the phenoxide ion.
3. Positional Influence:
Ortho and Para Substituents: Substituents at the ortho (o) and para (p) positions enhance acidity by facilitating resonance stabilization of the phenoxide ion.
Meta Substituents: Substituents at the meta (m) position are less effective in influencing acidity due to the lack of resonance stabilization.
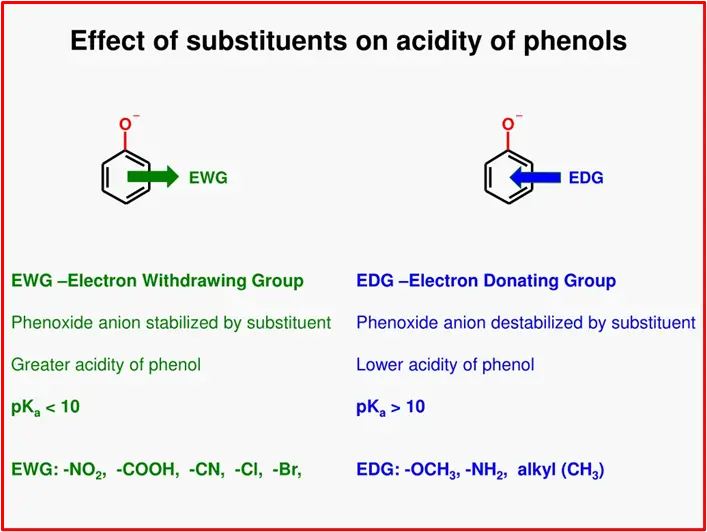
Conclusion:
In summary, electron-withdrawing substituents boost the acidity of phenol, while electron-donating substituents reduce acidity. The position of the substituent also plays a role, with ortho and para positions having a more significant impact on enhancing acidity. This understanding helps predict the relative acidity of substituted phenols in various chemical reactions.