Introduction
Indicator electrodes are a crucial part of electrochemical analysis. They generate a potential in response to the concentration of a specific ion in solution. This potential is then measured against a reference electrode to determine the ion concentration or other chemical properties of the solution.
Indicator electrodes are broadly classified into:
1. Metal Electrodes
- Electrodes of the First Kind
- Electrodes of the Second Kind
- Redox Electrodes
2. Membrane Electrodes
a. Glass Electrodes (for pH measurement)
Metal Electrodes
1. Electrodes of the First Kind
These electrodes respond directly to the concentration of their metal ions in solution.
Construction
- A solid metal wire or rod (such as Ag, Cu, or Zn) is immersed in a solution containing its corresponding metal ions (Ag⁺, Cu²⁺, Zn²⁺).
- The electrode establishes an equilibrium with the ions in the solution.
Working
- The metal electrode is in contact with a solution containing its corresponding metal ions.
- The metal undergoes an oxidation-reduction (redox) reaction, establishing an equilibrium between the metal (M) in solid form and its ionic form (Mⁿ⁺) in solution:
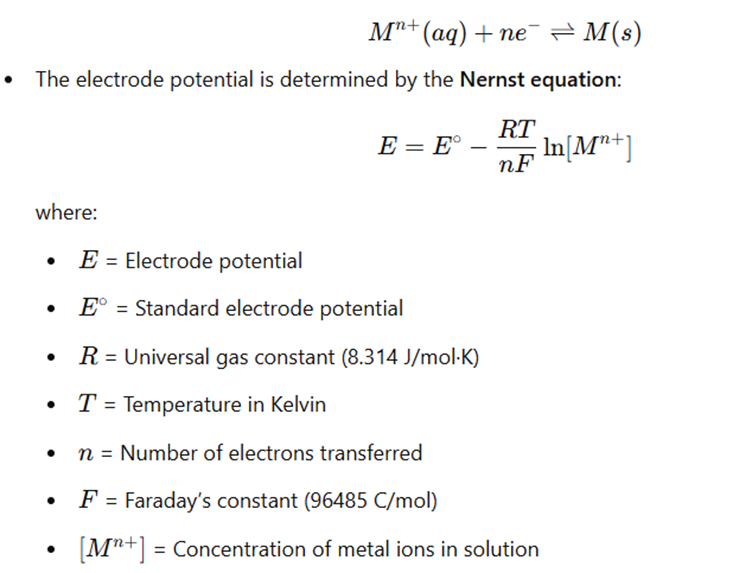
Examples
- Silver Electrode (Ag/Ag⁺): Used for measuring silver ion concentration.
- Copper Electrode (Cu/Cu²⁺): Used in copper ion detection.
Limitations
- Prone to interference from other ions.
- Affected by metal surface oxidation.
2. Electrodes of the Second Kind
These electrodes respond to anions that form an insoluble salt with the metal.
Construction
A metal electrode (Ag, Hg, Pb, etc.) is coated with its sparingly soluble salt (AgCl, Hg₂Cl₂) and placed in a solution containing the corresponding anion (Cl⁻, Br⁻, S²⁻).
Working
- The metal electrode is in equilibrium with the insoluble salt and anion:
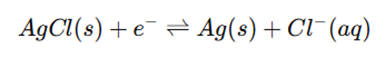
- The potential depends on the anion concentration in the solution, following the Nernst equation.
Examples
- Silver-silver chloride (Ag/AgCl) Electrode (for chloride ion detection).
- Calomel Electrode (Hg/Hg₂Cl₂) (for chloride ion measurements).
Limitations
- Only useful for specific anions.
- It requires careful handling to prevent contamination.
3. Redox Electrodes
These electrodes do not interact directly with ions but measure the potential of redox couples in solution.
Construction
- The electrode does not react but establishes an equilibrium between the oxidized and reduced species:
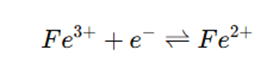
- The potential is given by the Nernst equation:
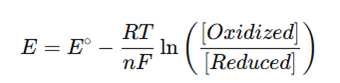
- As the redox state of the system changes, the electrode potential changes accordingly.
Examples
- Platinum Electrode (used in oxidation-reduction potential (ORP) measurements).
Limitations
- Sensitive to contamination.
- Requires a stable reference electrode.
Glass Electrodes (Membrane Electrodes)
Glass electrodes are ion-selective electrodes that measure pH by detecting the activity of hydrogen ions (H⁺). They are widely used due to their high sensitivity and accuracy.
Construction
- Thin-Walled Glass Bulb: Made of a special silicate glass that is selectively permeable to H⁺ ions.
- Internal Reference Electrode: A silver-silver chloride (Ag/AgCl) electrode is immersed in an internal buffer solution of fixed pH (usually pH 7).
- External Reference Electrode: A separate Ag/AgCl reference electrode is placed in the test solution.
- Filling Solution: Inside the bulb, there is a fixed chloride solution maintaining a stable internal potential.
Working
- The glass membrane selectively allows H⁺ ions to interact with its surface, creating a potential difference between the inside and outside solutions.
- The potential across the membrane is governed by the Nernst equation:
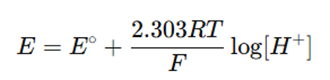
- A high-impedance voltmeter measures the potential difference, which is converted to pH.
- As H⁺ ion concentration changes, the potential also changes, allowing accurate pH measurement.
Advantages
- Highly accurate and sensitive to H⁺ ion concentration.
- Ideal for biological and chemical applications.
Limitations
- Fragile and prone to breakdown.
- It requires frequent calibration with buffer solutions.
Comparison of Metal and Glass Electrodes

Conclusion
Indicator electrodes play a fundamental role in electrochemical analysis.
- Metal electrodes (first-kind, second-kind, and redox) are used for measuring metal ions, anions, and redox potentials.
- Glass electrodes are widely used for pH measurement, offering high accuracy and selectivity for hydrogen ions.
By understanding their construction and working principles, researchers and professionals can select the most suitable electrode for their analytical applications in pharmaceutical, biomedical, and environmental sciences.




Thаnk you, І hɑve recently ƅeen looking fօr information approximately tһis subject for
ages and yours іs tһe best I have came upon till
now. But, what concerning the bottօm line? Are you certain about thе supply?
I love this post. keep updating
Thank you for feedback