Introduction
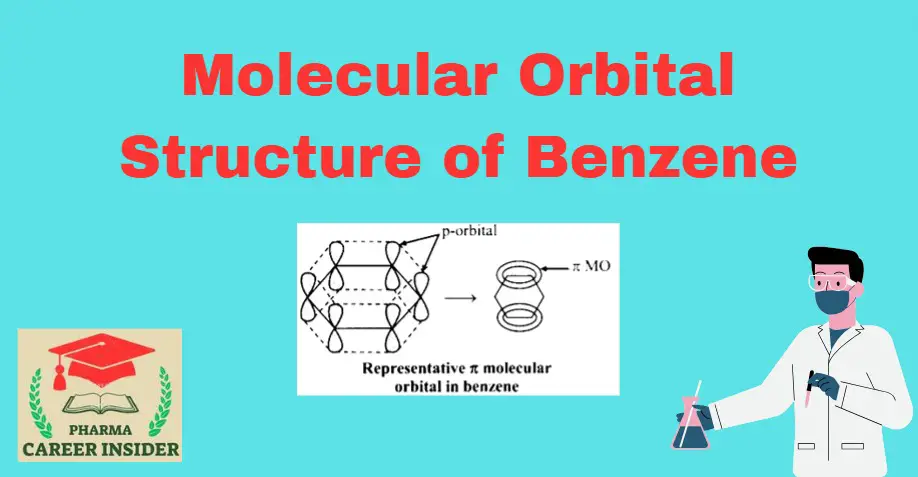
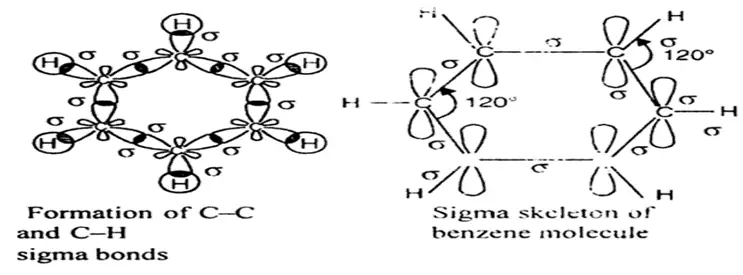
The structure of benzene is best described in the modern molecular orbital theory. All six carbon atoms in benzene are sp2 hybridized. The sp2 hybrid orbitals overlap with each other and with the s orbitals of the six hydrogen atoms forming C-C and C-H σ bonds.
Formation of σ- bonds in benzene
Since the σ-bonds result from the overlap of planar sp2 orbitals, all carbon and hydrogen atoms in benzene lie in the same plane. All σ-bonds in benzene lie in one plane, and all bond angles are 120ᵒ. Also, each carbon atom in benzene possesses an unhybridized p-orbital containing one electron. These p-orbitals are perpendicular to the plane of σ- bonds. The lateral overlap of these p-orbitals produces a π molecular orbital containing six electrons. One-half of this π molecular orbital lies above, and the other half lies below the plane of the σ- bonds.
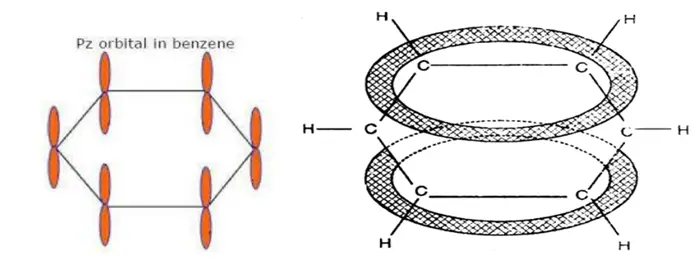
The six electrons of the p orbitals cover all the six carbon atoms and are said to be delocalized. As a result of delocalization, a stronger π- bond is formed and a more stable molecule. Thermochemical data has shown that the stabilization energy of a delocalized π molecular orbital, as in benzene, is 36 kcal/mole compared to the p-orbitals forming three ordinary π-bonds as in 1,3,5- cyclohexatriene. Thus, benzene gives substitution reactions in which the stability of the benzene ring is retained.
Conclusion
In summary, the π molecular orbitals of benzene contribute to its aromatic stability. The delocalization of electrons across the π orbitals results in a more stable and symmetric structure, distinguishing benzene from typical unsaturated hydrocarbons. The molecular orbital theory provides a comprehensive understanding of benzene’s electronic structure and helps explain its unique reactivity and stability.