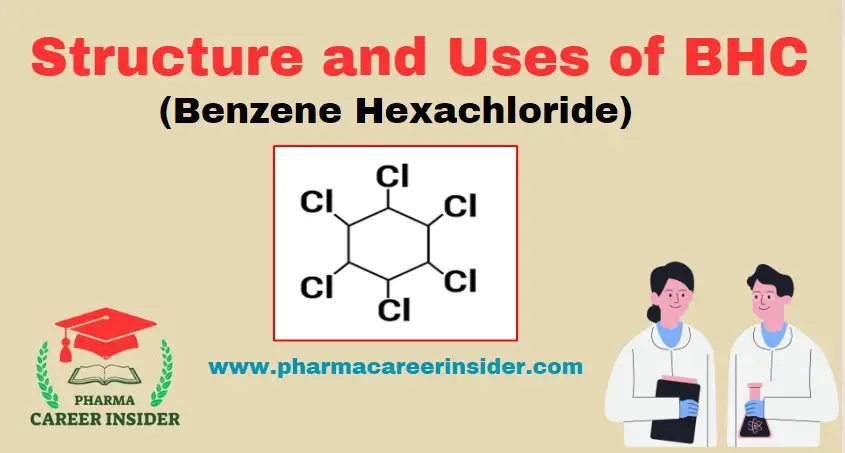
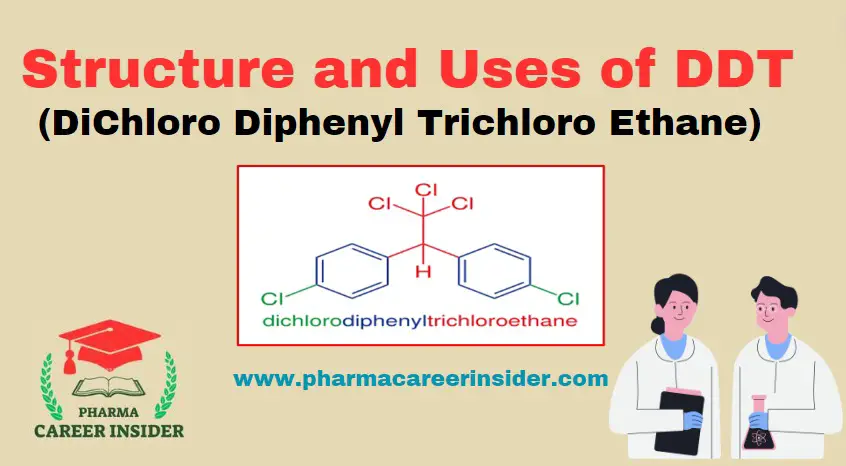
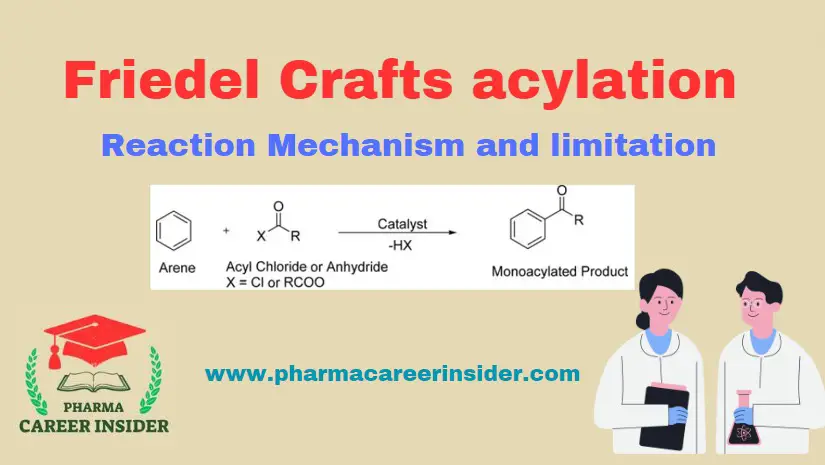
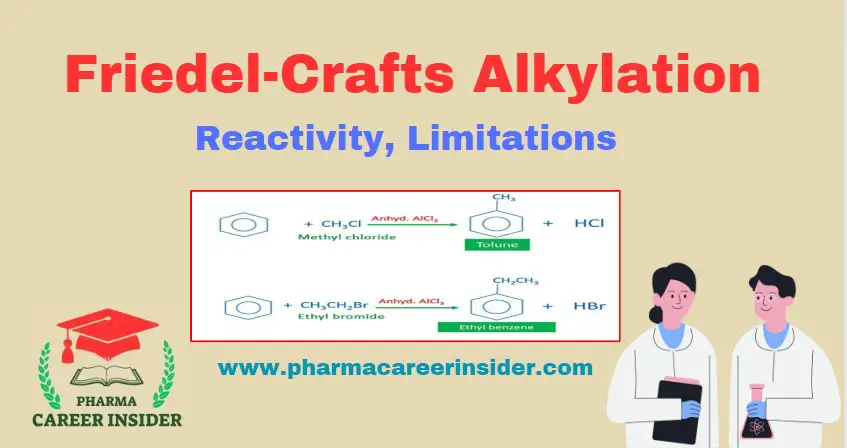
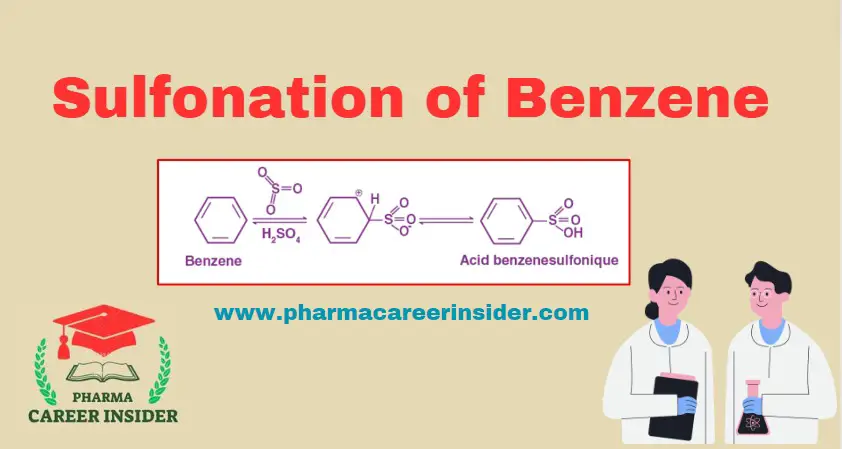
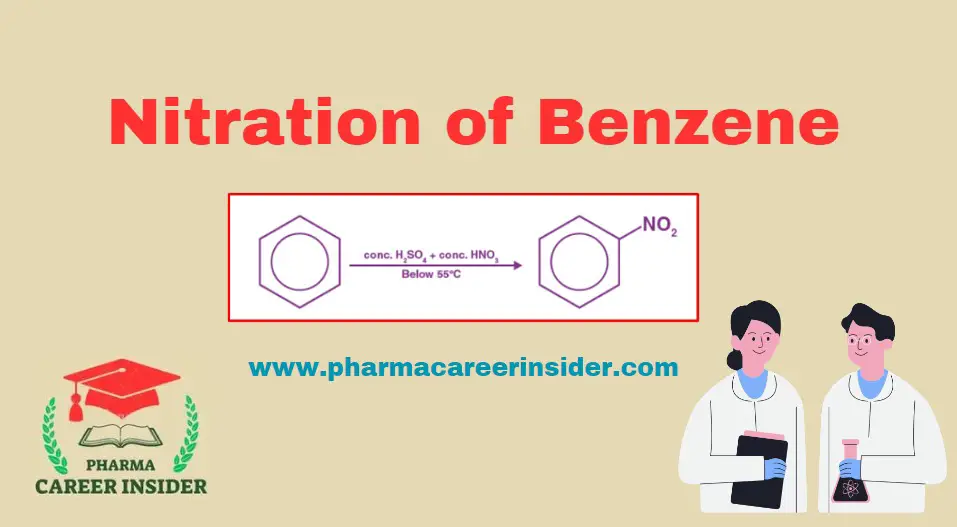
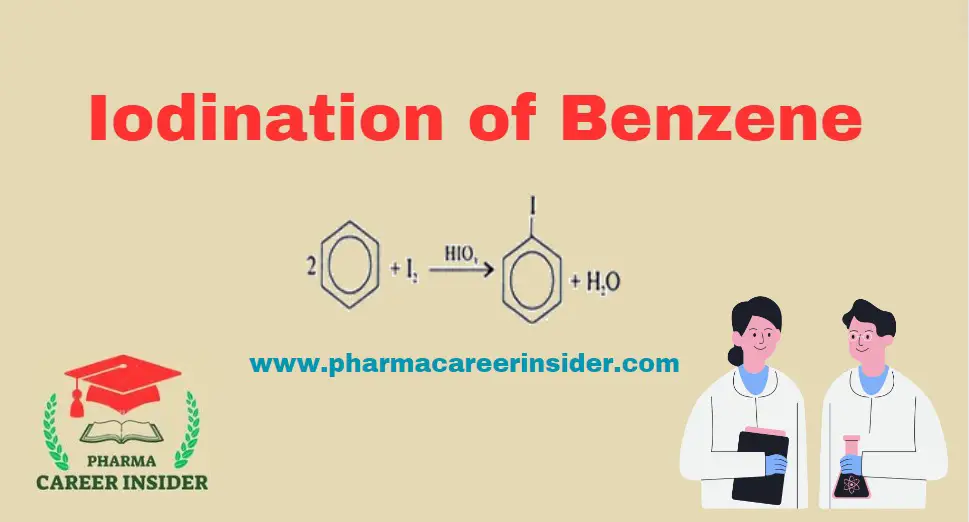
Structure and uses of BHC (Benzene Hexachloride)
Structure: Its IUPAC name is 1,2,3,4,5,6-hexachlorocyclohexane. Its molecular formula is C6H6 Cl6. Properties: BHC is a white, crystalline solid with no water solubility and is variable. Solubility in organic solvents. It is mostly soluble in halogenated solvents like chloroform, less. Soluble in esters and hydrocarbons and very less soluble in short-chain alcohols. Uses of BHC: