Meta Description
Learn about sodium bicarbonate (NaHCO₃), its preparation, assay, properties, and medicinal uses. Discover how it works as an antacid, urinary alkalizer, and pH regulator in medicine.
Introduction
Sodium bicarbonate (NaHCO₃), commonly known as baking soda, is a widely used antacid, alkalizing agent, and electrolyte replenisher. It plays an essential role in medicine, food processing, and industrial applications due to its acid-neutralizing and buffering properties. In healthcare, sodium bicarbonate is used for acid reflux, metabolic acidosis, and urinary alkalization.
This article explores the general methods of preparation, assay techniques, properties, and medicinal uses of sodium bicarbonate.
General Methods of Preparation
Sodium bicarbonate is prepared through various industrial and laboratory methods, with the most common being:
1. Solvay Process (Industrial Method)
The Solvay process is the most common method for large-scale sodium bicarbonate production. It involves the reaction of sodium chloride (NaCl), ammonia (NH₃), and carbon dioxide (CO₂) in water:

The sodium bicarbonate formed is filtered, washed, and dried for commercial use.
2. Carbonation of Sodium Carbonate (Laboratory Method)
Sodium bicarbonate can also be produced by bubbling carbon dioxide (CO₂) through a sodium carbonate (Na₂CO₃) solution:
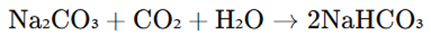
This method is commonly used for pharmaceutical-grade sodium bicarbonate production.
Assay of Sodium Bicarbonate
The purity of sodium bicarbonate is determined by acid-base titration using a standardized hydrochloric acid (HCl) solution.
Procedure
1. Dissolve a known quantity of sodium bicarbonate in distilled water.
2. Titrate with 0.1N hydrochloric acid (HCl) using methyl orange as an indicator.
3. The endpoint is reached when the solution changes from yellow to orange, indicating complete neutralization.
This assay ensures accurate determination of sodium bicarbonate concentration in pharmaceutical formulations.
Properties of Sodium Bicarbonate
Chemical Formula: NaHCO₃
Molecular Weight: 84.01 g/mol
Appearance: White crystalline powder
Solubility: Soluble in water, insoluble in alcohol
pH: Slightly alkaline (pH 8.3 in aqueous solution)
Odor & Taste: Odourless with a slightly salty taste
Stability: Decomposes to sodium carbonate (Na₂CO₃), water, and carbon dioxide upon heating
Medicinal Uses of Sodium Bicarbonate
Sodium bicarbonate is a versatile pharmaceutical agent used in various medical applications:
1. Antacid for Acid Reflux and Indigestion: Neutralizes excess stomach acid, providing relief from heartburn and acid reflux. Used in effervescent antacid formulations like Alka-Seltzer.
2. Treatment of Metabolic Acidosis: Administered in cases of metabolic acidosis due to kidney disease, diabetic ketoacidosis, or poisoning. It helps restore acid-base balance in critically ill patients.
3. Urinary Alkalizer: Used to alkalize urine, which helps in treating urinary tract infections (UTIs) and preventing kidney stones.They areenhancing the excretion of weakly acidic drugs like aspirin in cases of overdose.
4. Electrolyte Replenisher: Provides sodium ions essential for maintaining fluid and electrolyte balance in dehydration cases.Used in intravenous (IV) therapy for patients with severe acidosis.
5. Dental and Oral Care: Found in toothpaste and mouthwashes as a mild abrasive and pH regulator. Helps neutralize acidic conditions in the mouth, reducing plaque formation and bad breath.
6. Anti-Pruritic (Relief for Skin Irritation): Used in baking soda baths to relieve itching from insect bites, sunburns, and skin allergies.
Other Applications of Sodium Bicarbonate
Beyond medicine, sodium bicarbonate is widely used in various industries.
- Food Industry: Used as a leavening agent in baking (baking soda).
- Cleaning Agent: A natural deodorizer and surface cleaner.
- Fire Extinguishers: Used in dry chemical fire extinguishers.
- Water treatment: helps regulate pH in swimming pools and industrial wastewater.
Side Effects and Precautions
While sodium bicarbonate is generally safe, excessive use may cause:
1. Alkalosis (Excess Alkalinity): Symptoms include muscle twitching, confusion, and nausea.
2. Electrolyte imbalance: High sodium levels may cause hypertension and fluid retention.
3. Gastrointestinal Issues: Excess intake can lead to bloating, gas, and stomach cramps.
Precaution: Patients with hypertension, kidney disease, or heart conditions should consult a doctor before using sodium bicarbonate.
Conclusion
Sodium bicarbonate (NaHCO₃) is an essential antacid, alkalizing agent, and electrolyte replenisher used in medicine, food, and industry. It is widely used for acid reflux relief, metabolic acidosis treatment, urinary alkalization, and electrolyte balance. Due to its versatility and safety, sodium bicarbonate remains a vital pharmaceutical and industrial compound. However, controlled use is necessary to prevent potential side effects like alkalosis and electrolyte imbalances.
Frequently Asked Questions (FAQs)
1. How is sodium bicarbonate prepared?
Answer: Sodium bicarbonate is produced using the Solvay process or by carbonation of sodium carbonate (Na₂CO₃) with carbon dioxide (CO₂).
2. What are the main medicinal uses of sodium bicarbonate?
Answer: It is used as an antacid, urinary alkalizer, metabolic alkalosis corrector, and electrolyte replenisher.
3. Can sodium bicarbonate be used daily?
Answer: While occasional use is safe, long-term or excessive use can cause alkalosis, electrolyte imbalances, and gastrointestinal issues




