Jan
04
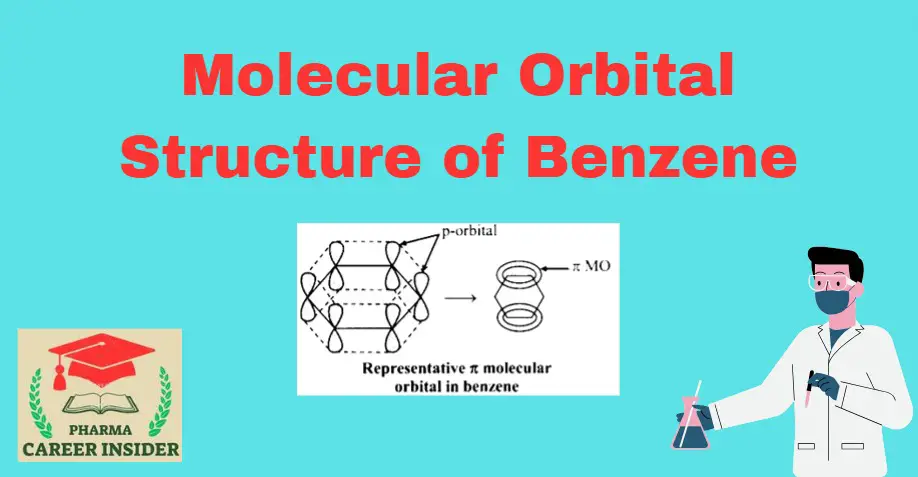
Introduction The structure of benzene is best described in the modern molecular orbital theory. All six carbon atoms in benzene are sp2 hybridized. The sp2 hybrid orbitals overlap with each other and with the s orbitals of the six hydrogen atoms forming C-C and C-H σ bonds. Formation of σ- bonds in benzene Since the … Read more