Complexometric titration is based on the formation of a stable complex between a metal ion (analyte) and a chelating agent (titrant). The process involves the quantitative determination of metal ions in a solution by measuring the amount of titrant required to form a complete complex with the metal ions.
Fundamental Concepts:
- Metal-Ligand Interaction: A central metal ion (Lewis’s acid) reacts with a chelating agent (Lewis’s base) to form a coordination complex through coordinate covalent bonds.
- Chelation Process: Chelation occurs when the chelating agent binds to the metal ion at multiple sites, forming a highly stable ring-like structure called a chelate. This increases the stability of the resulting complex compared to complexes formed by monodentate ligands.
- Endpoint Detection: The endpoint of the titration is determined by using a metal ion indicator, which changes color when all the metal ions have been complexed. The indicator forms a less stable complex with the metal ion, which is displaced by the chelating agent.
- pH Dependence: The stability of the metal-ligand complex is pH-dependent. Buffer solutions are often used to maintain a specific pH range, ensuring optimal complex formation.
Reaction Mechanism:
- The general reaction for complexometric titration using EDTA (a hexadentate ligand) as a titrant can be represented as:
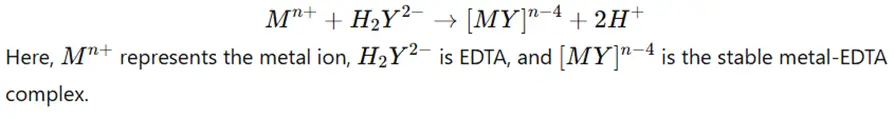
Important Parameters:
- Stability Constant (Kf): A measure of the stability of the complex. Higher Kf values indicate a more stable complex, ensuring accurate titration results.
- Equivalence Point: The point at which the moles of titrant added are stoichiometrically equivalent to the moles of metal ions in the solution.
- Indicator Action: The metal ion indicator exhibits a distinct color change when displaced by the chelating agent, signaling the endpoint.
Applications:
- Determination of metal ions such as calcium, magnesium, zinc, and lead.
- Water hardness analysis.
- Pharmaceutical analysis for compounds like magnesium sulfate and calcium gluconate.



